Neuac Kit
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%
FULL PRESCRIBING INFORMATION: CONTENTS*
- NEUAC KIT DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- NEUAC KIT INDICATIONS AND USAGE
- NEUAC KIT CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- NEUAC KIT ADVERSE REACTIONS
- NEUAC KIT DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - Kit Carton
FULL PRESCRIBING INFORMATION
Rx Only
For Dermatological Use Only.
Not for Ophthalmic Use.
NEUAC KIT DESCRIPTION
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% contains clindamycin phosphate, (7(S)-chloro-7-deoxylincomycin-2-phosphate), equivalent to 1% clindamycin, and 5% benzoyl peroxide.
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin.
Clindamycin phosphate is C18H34CIN2O8PS. The structural formula for clindamycin phosphate is represented below:
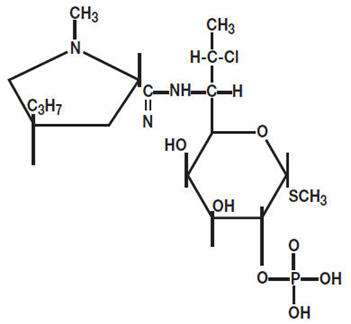
Clindamycin phosphate has a molecular weight of 504.97 and its chemical name is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate)
Benzoyl peroxide is C14H10O4. It has the following structural formula:
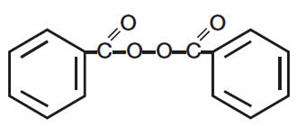
Benzoyl peroxide has a molecular weight of 242.23.
Each gram of Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% contains 10 mg (1%) clindamycin, as phosphate, and 50 mg (5%) benzoyl peroxide in a base consisting of carbomer homopolymer (type B), hydrochloric acid, methylparaben, dimethicone, propylparaben, purified water and sodium hydroxide.
CLINICAL PHARMACOLOGY
A comparative study of the pharmacokinetics of clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% and 1% clindamycin solution alone in 78 patients indicated that mean plasma clindamycin levels during the four week dosing period were < 0.5 ng/ml for both treatment groups.
Benzoyl peroxide has been shown to be absorbed by the skin where it is converted to benzoic acid. Less than 2% of the dose enters systemic circulation as benzoic acid.
Microbiology
Mechanism of Action
Clindamycin binds to the 50S ribosomal subunits of susceptible bacteria and prevents elongation of peptide chains by interfering with peptidyl transfer, thereby suppressing protein synthesis.
Benzoyl peroxide is a potent oxidizing agent.
In Vivo Activity
No microbiology studies were conducted in the clinical trials with this product.
In Vitro Activity
The clindamycin and benzoyl peroxide components individually have been shown to have in vitro activity against Propionibacterium acnes, an organism which has been associated with acne vulgaris; however, the clinical significance of this is not known.
Drug Resistance
There are reports of an increase of P. acnes resistance to clindamycin in the treatment of acne. In patients with P. acnes resistant to clindamycin, the clindamycin component may provide no additional benefit beyond benzoyl peroxide alone.
CLINICAL STUDIES
In five randomized, double-blind clinical studies of 1,319 patients, 397 used clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%, 396 used benzoyl peroxide, 349 used clindamycin and 177 used vehicle. Clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% applied once daily for 11 weeks was significantly more effective than vehicle, benzoyl peroxide, and clindamycin in the treatment of inflammatory lesions of moderate to moderately severe facial acne vulgaris in three of the five studies (Studies 1, 2, and 5).
Patients were evaluated and acne lesions counted at each clinical visit: weeks 2, 5, 8, 11. The primary efficacy measures were the lesion counts and the investigator's global assessment evaluated at week 11. Patients were instructed to wash the face, wait 10 to 20 minutes, and then apply medication to the entire face, once daily, in the evening before retiring. Percent reductions in inflammatory lesion counts after treatment for 11 weeks in these five studies are shown in the following table:
| Study 1 (n=120) |
Study 2 (n=273) |
Study 3 (n=280) |
Study 4 (n=288) |
Study 5 (n=358) |
|
|---|---|---|---|---|---|
| Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% | 65% | 56% | 42% | 57% | 52% |
| Benzoyl Peroxide | 36% | 37% | 32% | 57% | 41% |
| Clindamycin | 34% | 30% | 38% | 49% | 33% |
| Vehicle | 19% | -0.4% | 29% | -- | 29% |
The clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% group showed greater overall improvement in the investigator's global assessment than the benzoyl peroxide, clindamycin and vehicle groups in three of the five studies (Studies 1, 2, and 5).
Clinical studies have not adequately demonstrated the effectiveness of clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% versus benzoyl peroxide alone in the treatment of non-inflammatory lesions of acne.
NEUAC KIT INDICATIONS AND USAGE
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is indicated for the topical treatment of inflammatory acne vulgaris.
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% has not been demonstrated to have any additional benefit when compared to benzoyl peroxide alone in the same vehicle when used for the treatment of non-inflammatory acne.
NEUAC KIT CONTRAINDICATIONS
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is contraindicated in those individuals who have shown hypersensitivity to any of its components or to lincomycin. It is also contraindicated in those having a history of regional enteritis, ulcerative colitis, pseudomembranous colitis, or antibiotic-associated colitis.
WARNINGS
ORALLY AND PARENTERALLY ADMINISTERED CLINDAMYCIN HAS BEEN ASSOCIATED WITH SEVERE COLITIS WHICH MAY RESULT IN PATIENT DEATH. USE OF THE TOPICAL FORMULATION OF CLINDAMYCIN RESULTS IN ABSORPTION OF THE ANTIBIOTIC FROM THE SKIN SURFACE. DIARRHEA, BLOODY DIARRHEA, AND COLITIS (INCLUDING PSEUDOMEMBRANOUS COLITIS) HAVE BEEN REPORTED WITH THE USE OF TOPICAL AND SYSTEMIC CLINDAMYCIN. STUDIES INDICATE A TOXIN(S) PRODUCED BY CLOSTRIDIA IS ONE PRIMARY CAUSE OF ANTIBIOTIC-ASSOCIATED COLITIS. THE COLITIS IS USUALLY CHARACTERIZED BY SEVERE PERSISTENT DIARRHEA AND SEVERE ABDOMINAL CRAMPS AND MAY BE ASSOCIATED WITH THE PASSAGE OF BLOOD AND MUCUS. ENDOSCOPIC EXAMINATION MAY REVEAL PSEUDOMEMBRANOUS COLITIS. STOOL CULTURE FOR Clostridium difficile AND STOOL ASSAY FOR Clostridium difficile TOXIN MAY BE HELPFUL DIAGNOSTICALLY. WHEN SIGNIFICANT DIARRHEA OCCURS, THE DRUG SHOULD BE DISCONTINUED. LARGE BOWEL ENDOSCOPY SHOULD BE CONSIDERED TO ESTABLISH A DEFINITIVE DIAGNOSIS IN CASES OF SEVERE DIARRHEA. ANTIPERISTALTIC AGENTS SUCH AS OPIATES AND DIPHENOXYLATE WITH ATROPINE MAY PROLONG AND/OR WORSEN THE CONDITION. DIARRHEA, COLITIS AND PSEUDOMEMBRANOUS COLITIS HAVE BEEN OBSERVED TO BEGIN UP TO SEVERAL WEEKS FOLLOWING CESSATION OF ORAL AND PARENTERAL THERAPY WITH CLINDAMYCIN.
Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
PRECAUTIONS
General
For dermatological use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents.
The use of antibiotic agents may be associated with the overgrowth of nonsusceptible organisms, including fungi. If this occurs, discontinue use of this medication and take appropriate measures.
Avoid contact with eyes and mucous membranes.
Clindamycin and erythromycin containing products should not be used in combination. In vitro studies have shown antagonism between these two antimicrobials. The clinical significance of this in vitro antagonism is not known.
Information for Patients
Patients using Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should receive the following information and instructions:
- Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is to be used as directed by the physician. It is for external use only. Avoid contact with eyes, and inside the nose, mouth, and all mucous membranes, as this product may be irritating.
- This medication should not be used for any disorder other than that for which it was prescribed.
- Patients should not use any other topical acne preparation unless otherwise directed by their physician.
- Patients should report any signs of local adverse reactions to their physician. Patients who develop allergic symptoms such as severe swelling or shortness of breath should discontinue use and contact their physician immediately.
- Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% may bleach hair or colored fabric.
- Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% can be stored at room temperature up to 25°C (77°F) for up to 2 months. Do not freeze. Keep tube tightly closed. Keep out of the reach of small children. Discard any unused product after 2 months.
- Before applying Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% to affected areas, wash the skin gently, rinse with warm water, and pat dry.
- Excessive or prolonged exposure to sunlight should be limited. To minimize exposure to sunlight, a hat or other clothing should be worn.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Benzoyl peroxide has been shown to be a tumor promoter and progression agent in a number of animal studies. Benzoyl peroxide in acetone at doses of 5 and 10 mg administered twice per week induced squamous cell skin tumors in transgenic TgAC mice in a study using 20 weeks of topical treatment. The clinical significance of this is unknown.
In a 2-year dermal carcinogenicity study in mice, treatment with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% at doses up to 8000 mg/kg/day (16 times the highest recommended adult human dose of 2.5 g clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%, based on mg/m2) did not cause an increase in skin tumors. However, topical treatment with another formulation containing 1% clindamycin and 5% benzoyl peroxide at doses of 100, 500, or 2000 mg/kg/day caused a dose-dependent increase in the incidence of keratoacanthoma of the treated skin site of male rats in a 2-year dermal carcinogenicity study in rats.
In a 52-week photocarcinogenicity study in hairless mice (40 weeks of treatment followed by 12 weeks of observation), the median time to onset of skin tumor formation decreased and the number of tumors per mouse increased relative to controls following chronic concurrent topical treatment with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% and exposure to ultraviolet radiation.
Genotoxicity studies were not conducted with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%. Clindamycin phosphate was not genotoxic in Salmonella typhimurium or in a rat micronucleus test. Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian cell types, to be mutagenic in Salmonella typhimurium tests by some but not all investigators, and to cause sister chromatid exchanges in Chinese hamster ovary cells.
Studies have not been performed with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% or benzoyl peroxide to evaluate the effect on fertility. Fertility studies in rats treated orally with up to 300 mg/kg/day of clindamycin (approximately 120 times the amount of clindamycin in the highest recommended adult human dose of 2.5 g clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%, based on mg/m2) revealed no effects on fertility or mating ability.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% or benzoyl peroxide. It is also not known whether clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should be given to a pregnant woman only if clearly needed.
Developmental toxicity studies performed in rats and mice using oral doses of clindamycin up to 600 mg/kg/day (240 and 120 times the amount of clindamycin in the highest recommended adult human dose based on mg/m2, respectively) or subcutaneous doses of clindamycin up to 250 mg/kg/day (100 and 50 times the amount of clindamycin in the highest recommended adult human dose based on mg/m2, respectively) revealed no evidence of teratogenicity.
Nursing Women
It is not known whether Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is secreted into human milk after topical application. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of this product in pediatric patients below the age of 12 have not been established.
NEUAC KIT ADVERSE REACTIONS
During clinical trials, all patients were graded for facial erythema, peeling, burning, and dryness on the following scale: 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. The percentage of patients that had symptoms present before treatment (at baseline) and during treatment were as follows:
| Local reactions with use of clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% % of patients using clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% with symptom present Combined results from 5 studies (n=397) |
||||||
|---|---|---|---|---|---|---|
| Before Treatment (Baseline) | During Treatment | |||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | |
| (Percentages derived by # subjects with symptom score/# enrolled clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% subjects, n = 397). | ||||||
| Erythema | 28% | 3% | 0 | 26% | 5% | 0 |
| Peeling | 6% | <1% | 0 | 17% | 2% | 0 |
| Burning | 3% | <1% | 0 | 5% | <1% | 0 |
| Dryness | 6% | <1% | 0 | 15% | 1% | 0 |
Anaphylaxis, as well as allergic reactions leading to hospitalization, has been reported in postmarketing use with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
NEUAC KIT DOSAGE AND ADMINISTRATION
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should be applied once daily, in the evening or as directed by the physician, to affected areas after the skin is gently washed, rinsed with warm water and patted dry.
HOW SUPPLIED
Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is available in a 45 gram tube (NDC 43538-176-45).
Prior to Dispensing
Store in a cold place, preferably in a refrigerator, between 2°C and 8°C (36°F and 46°F). Do not freeze.
Dispensing Instructions for the Pharmacist
Dispense Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% with a 60 day expiration date and specify "Store at room temperature up to 25°C (77°F). Do not freeze."
Keep tube tightly closed. Keep out of the reach of small children.
Manufactured for Medimetriks Pharmaceuticals, Inc.
383 Route 46 West, Fairfield, NJ 07004-2402 USA
www.medimetriks.com
Manufactured by Perrigo,Yeruham 80500, Israel • Made in Israel
Iss. 03/14
IP037
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 43538-177-45
Rx Only
Neuac ™ KIT
(clindamycin phosphate and
benzoyl peroxide) Gel, 1.2%/5%
For External Use Only
KIT CONTENTS:
1 - Neuac™ (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% - 45 g
1 - Niseko™ Hydrating Facial Moisturizer - Net wt. 3 oz. (85 g)
MEDIMETRIKS
PHARMACEUTICAL, INC.

Neuac Kitclindamycin phosphate and benzoyl peroxide KIT
| ||||||||||||||||||||||||||||||||||||||||