Neutrogena Corporation
Neutrogena Healthy Skin Compact Makeup with Sunscreen Broad Spectrum SPF 55
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredients
Avobenzone 2.8%, Homosalate 8%, Octisalate 4%, Octocrylene 9%, Oxybenzone 4%
Purpose
Sunscreen
Neutrogena Healthy Skin Compact Makeup Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see
Directions
), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
-
Do not use on damaged or broken skin
-
When using this product keep out of eyes. Rinse with water to remove.
-
Stop use and ask a doctor if rash occurs
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
Other Information
- protect this product from excessive heat and direct sun
- may stain some fabrics
Inactive Ingredients
Isononyl Isononanoate, Silica, Beeswax, Mica, C12-15 Alkyl Benzoate, Calcium Aluminum Borosilicate, Styrene/Acrylates Copolymer, Polyethylene, Diethylhexyl 2,6-Naphthalate, VP/Hexadecene Copolymer, Dimethicone/Vinyl Dimethicone Crosspolymer, Dimethicone, Acrylates/Dimethicone Copolymer, Phenoxyethanol, Caprylyl Glycol, Bisabolol, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Polymethyl Methacrylate, Triethoxycaprylylsilane, Tocopheryl Acetate, Pantothenic Acid, Retinyl Palmitate, Ascorbic Acid May Contain: Titanium Dioxide, Iron Oxides
Questions or Comments?
Call toll-free 800-480-4812 or 215-273-8755 (collect) or visit www.neutrogena.com
Dist. by Neutrogena Corporation, Los Angeles, CA 90045
PRINCIPAL DISPLAY PANEL - 9.9g Container Label - CLASSIC IVORY 10
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®

PRINCIPAL DISPLAY PANEL - 9.9g Container Label - NATURAL IVORY 20
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®

PRINCIPAL DISPLAY PANEL - 9.9g Container Label - BUFF 30
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®

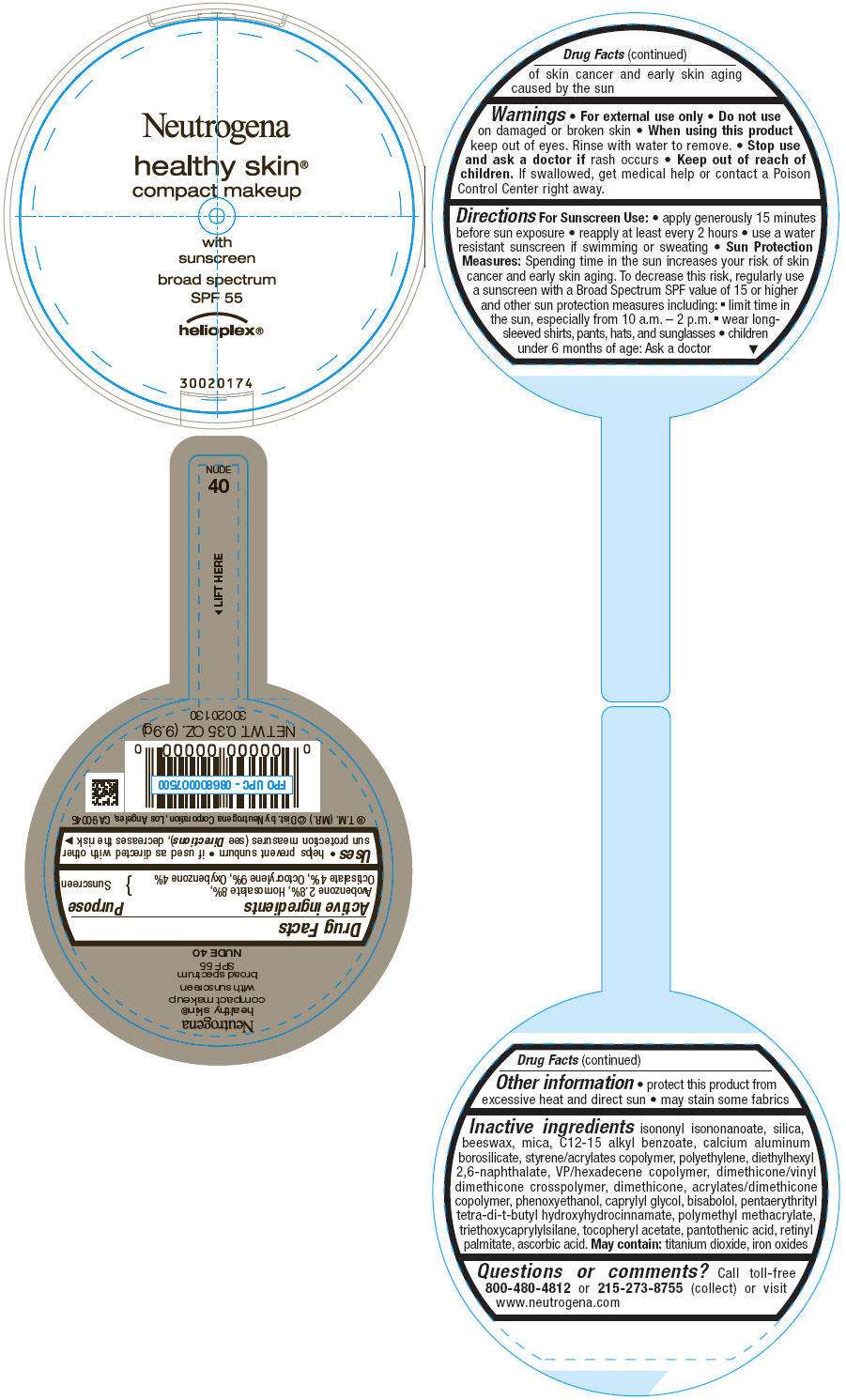
PRINCIPAL DISPLAY PANEL - 9.9g Container Label - NUDE 40
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
PRINCIPAL DISPLAY PANEL - 9.9g Container Label - SOFT BEIGE 50
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®

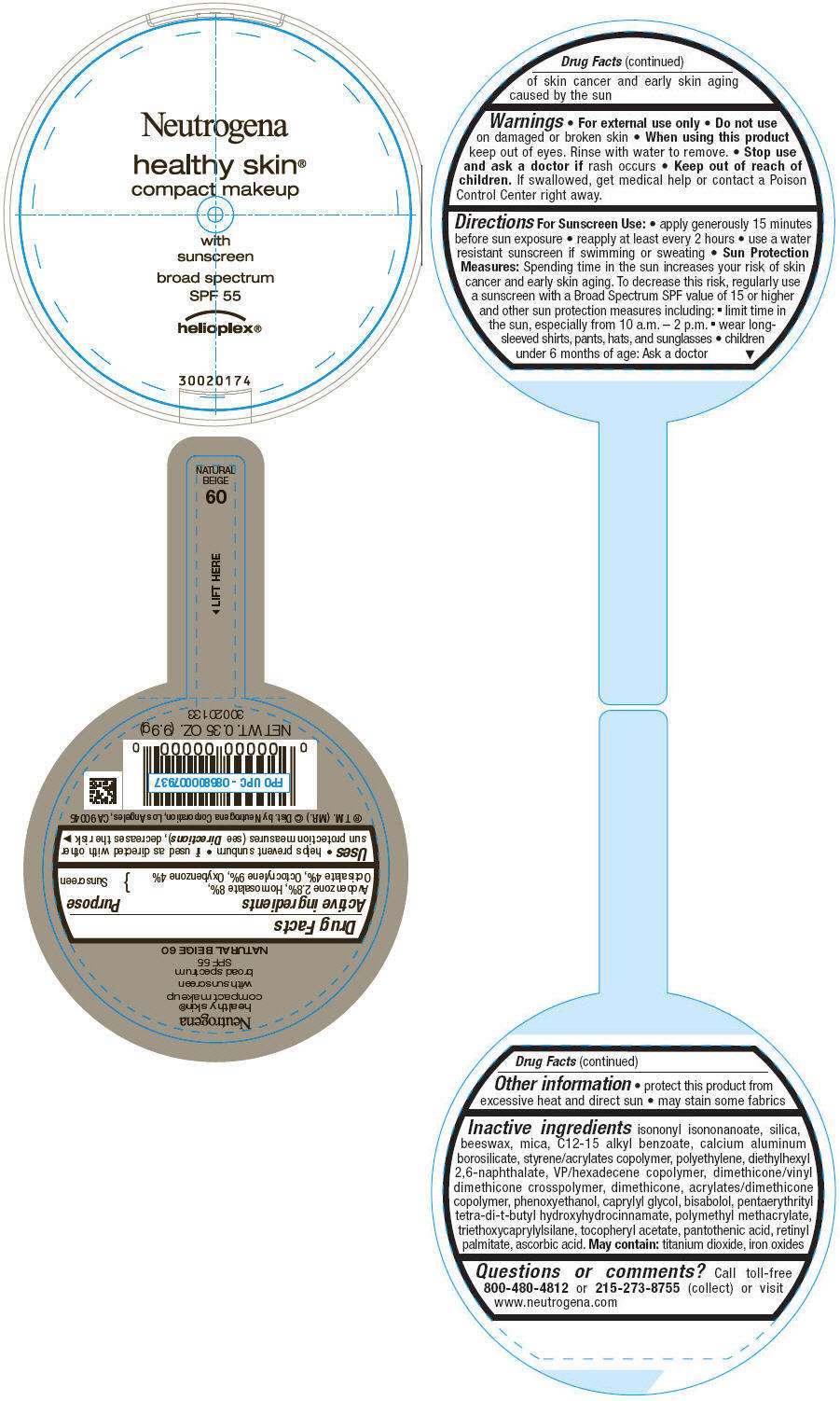
PRINCIPAL DISPLAY PANEL - 9.9g Container Label - NATURAL BEIGE 60
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
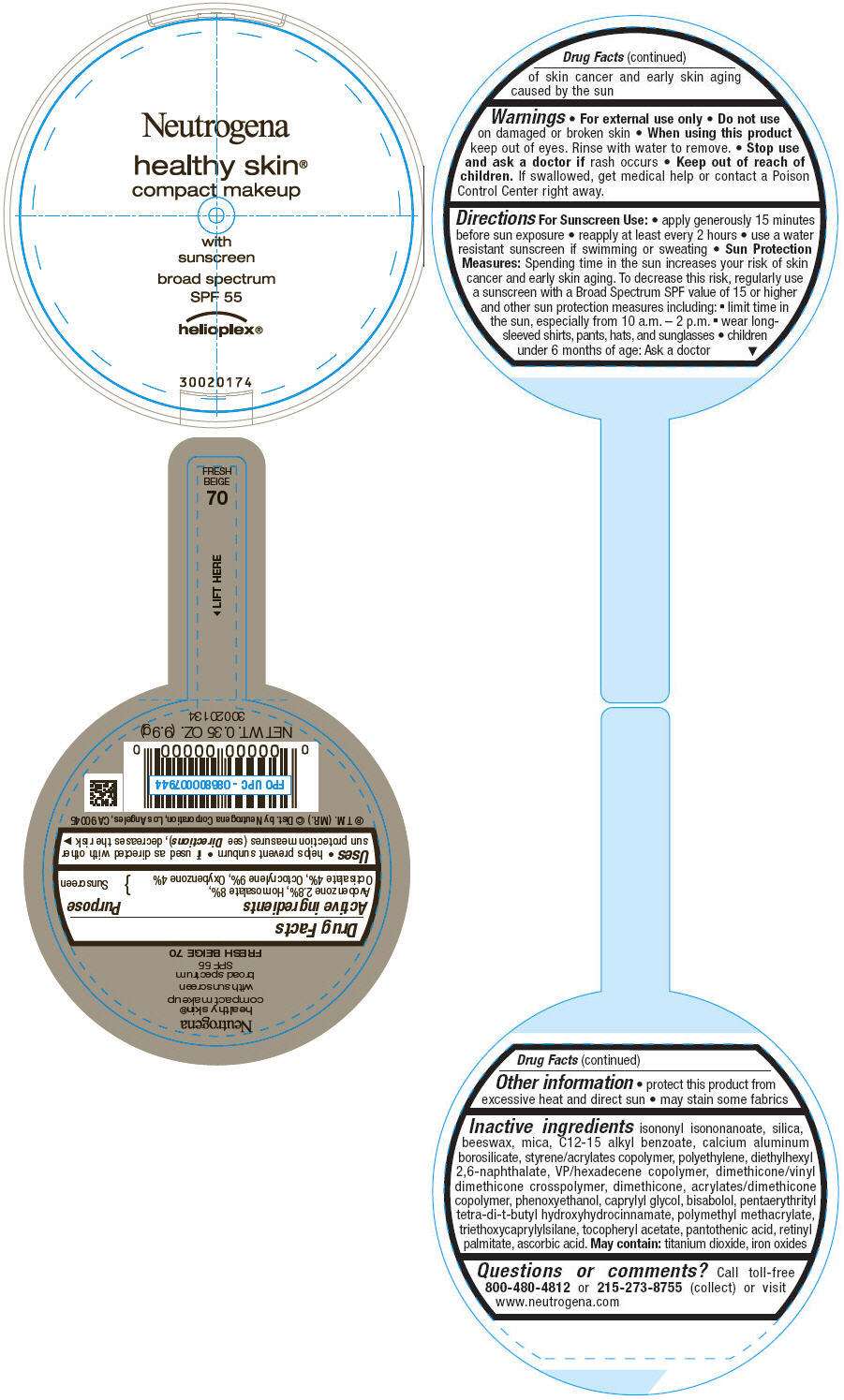
PRINCIPAL DISPLAY PANEL - 9.9g Container Label - FRESH BEIGE 70
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®
PRINCIPAL DISPLAY PANEL - 9.9g Container Label - WARM BEIGE 90
Neutrogena
healthy skin®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex®

Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-091 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-091-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-092 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-092-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-093 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-093-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-094 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-094-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-095 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-095-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-096 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-096-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-097 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-097-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|
Neutrogena Healthy Skin Compact Makeup
Avobenzone, Homosalate, Octisalate, Octocrylene, and Oxybenzone CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-098 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-098-01 |
9.9 in 1 CONTAINER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-12-06 |
|
|







