No7 Time Resisting Day Sunscreen SPF 12
Carton and jar labels
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients Purpose
Purpose
Uses- Provides moderate protection against adverse effects of the sun
- Higher SPF gives more protection
Warnings
For external use only
When using this product
Avoid contact with eyes. If contact occurs rinse immediately with cold water.
Stop use and ask a doctor if rash or irritation develops and lasts.
Keep out of reach of children.
Uses
- After cleansing and toning, massage onto face and neck.
oo
Inactive ingredients
Aqua (Water), Butylene glycol, C12-15 alkyl benzoate, Sorbitan stearate, Dimethicone, Isohexadecane, Cetyl alcohol, Prunus amygdalus dulcis (Sweet almond oil), Mica, Phenoxyethanol, Polyacrylamide, Glyceryl stearate, C18-36 acid glycol ester, Tocopheryl acetate, PEG-100 stearate, C13-14 isoparaffin, Parfum (Fragrance), Dimethiconol, Sucrose cocoate, Methylparaben, Sodium ascorbyl phosphate, Sodium stearoyl lactylate, Carbomer, Propylparaben, Laureth-7, Tetrasodium EDTA, Potassium hydroxide, Propylene glycol, Glycerin, Xanthan gum, Retinyl palmitate, Panax ginseng root extract, BHT, Morus alba leaf extract, Sodium hydroxide, Tocopherol, CI 77891 (Titanium dixoide).
USA: Questions? 1-866-75-BOOTS
Active ingredient
Boots
Mfd in the UK for BBI Ltd NG2 3AA. UK
Dist in the USA by Boots Retail USA Inc. Stamford CT 06901
No7
Time Resisting
Day Cream
Younger looking skin in just 2 weeks
Sunscreen SPF 12
Hypo-allergenic
Boots
50ml e 1.69 US Fl. Oz
Time Resisting Day Cream carton.jpg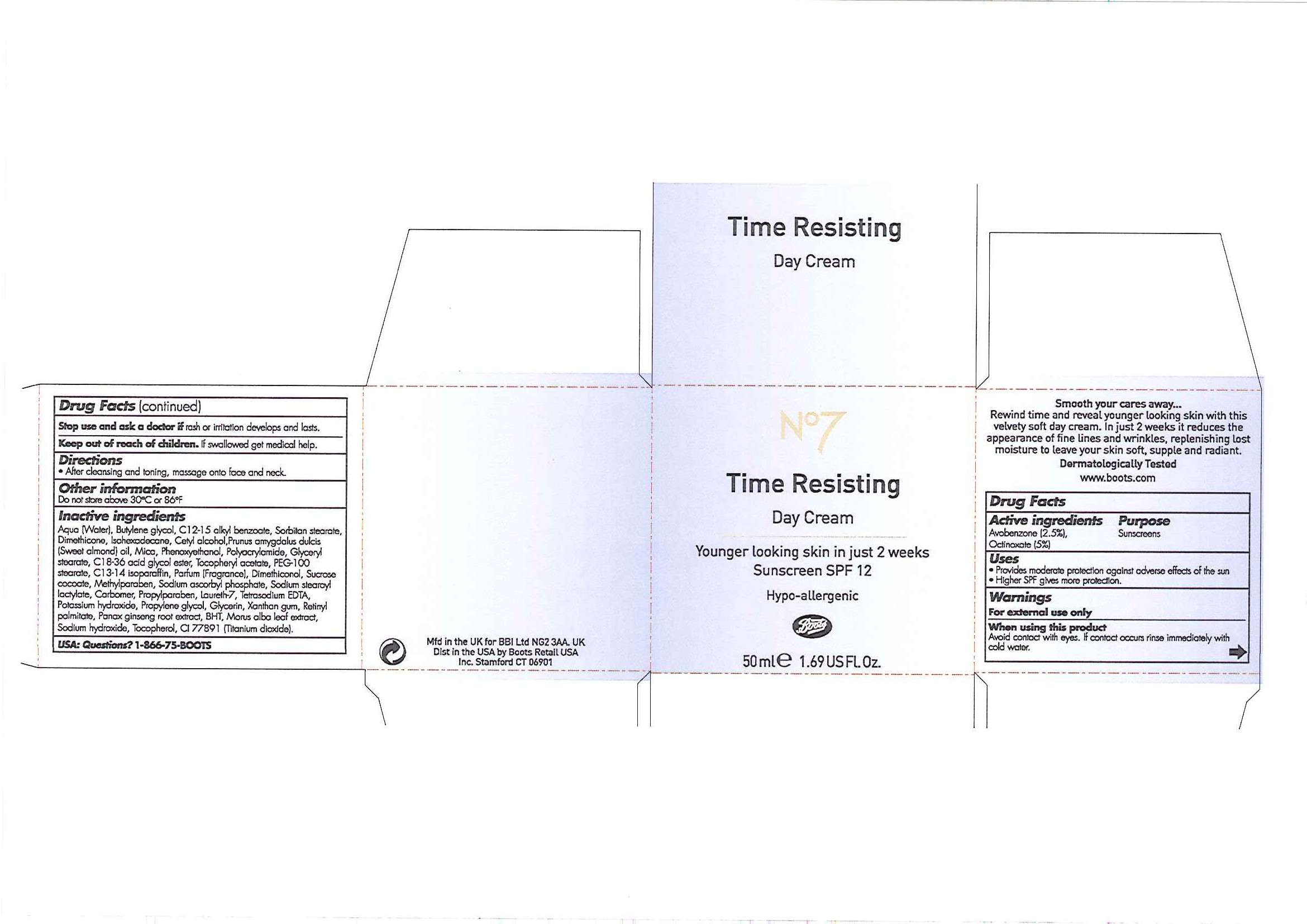
Time Resisting
Day Cream
Sunscreen SPF 12
50ml 1.69 US Fl. Oz
Time Resisting Day Cream jar.jpg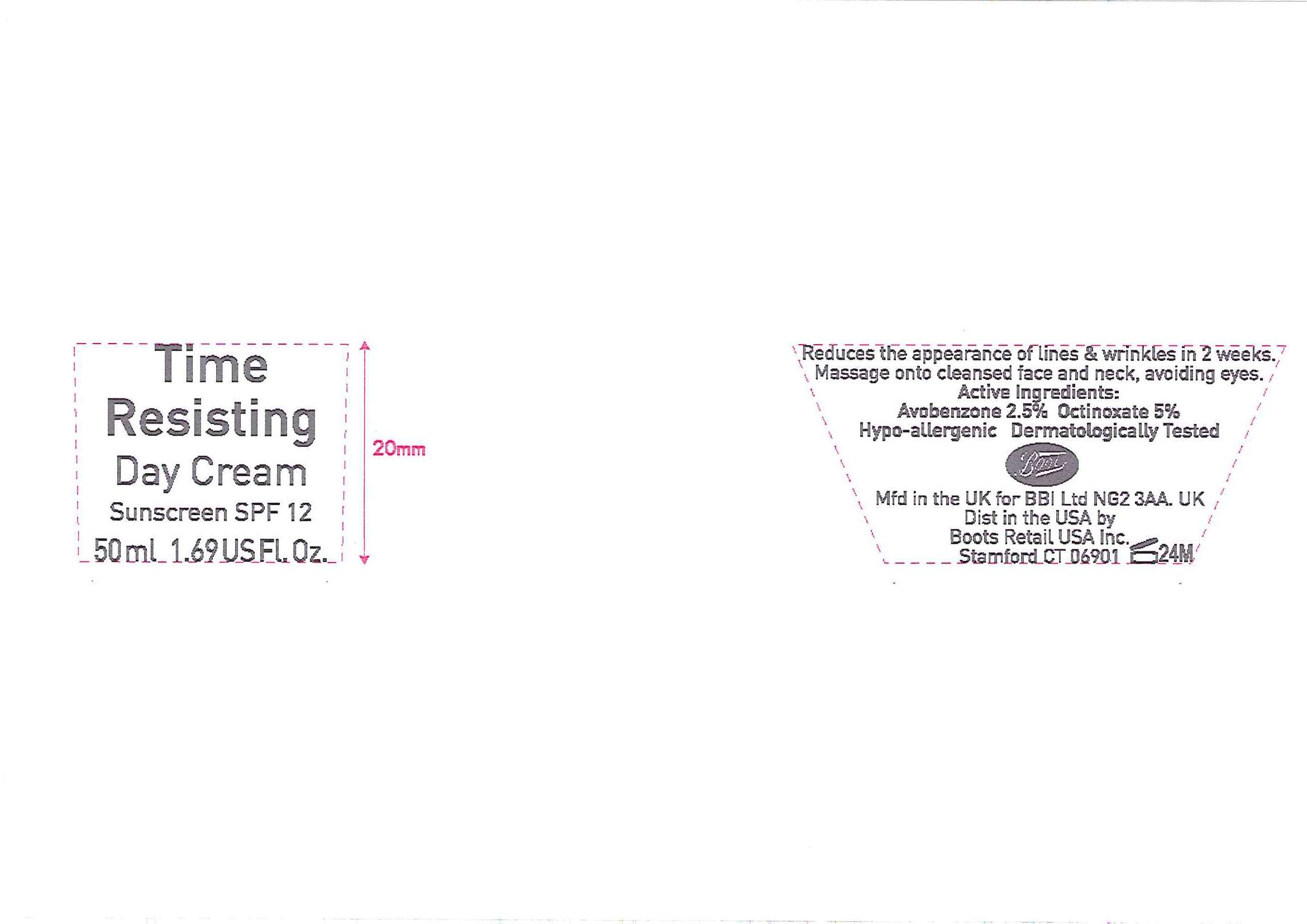
No7 Time Resisting Day Sunscreen SPF 12Octinoxate and Avobenzone CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||