Nystatin
Nystatin, USPFor Extemporaneous Preparation of Oral Suspension
FULL PRESCRIBING INFORMATION: CONTENTS*
- NYSTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS FOR USAGE
- NYSTATIN CONTRAINDICATIONS
- NYSTATIN ADVERSE REACTIONS
- NYSTATIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
NYSTATIN DESCRIPTION
Nystatin USP is an antifungal antibiotic obtained from Streptomyces noursei. It is known to be a mixture, but the composition has not been completely elucidated. Nystatin A is closely related to amphotericin B. Each is a macrocyclic lactone containing a ketal ring, an all-trans polyene system, and a mycosamine (3-amino-3-deoxy-rhamnose) moiety. Nystatin A has a molecular formula of C47H75NO17 and a molecular weight of 926.11.
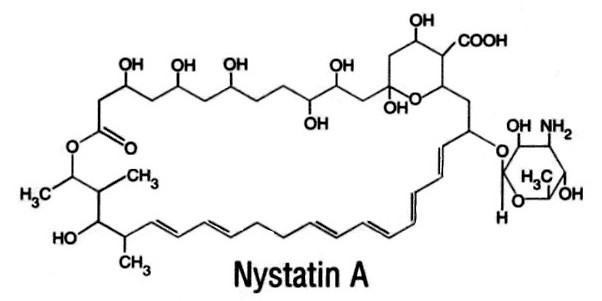
Nystatin USP is a ready-to-use, non-sterile powder for oral administration which contains no excipients or preservatives. It is available in containers of 50 million, 150 million, 500 million, and 2 billion units. Each mg contains a minimum of 5,000 units.
CLINICAL PHARMACOLOGY
Nystatin probably acts by binding to sterols in the cell membrane of the fungus with a resultant change in membrane permeability allowing leakage of intracellular components. It is absorbed very sparingly following oral administration, with no detectable blood levels when given in the recommended doses. Most of the orally administered nystatin is passed unchanged in the stool.
INDICATIONS FOR USAGE
For the treatment of intestinal and oral cavity infections caused by Candida (Monilia) albicans.
NYSTATIN CONTRAINDICATIONS
Hypersensitivity to the drug.
NYSTATIN ADVERSE REACTIONS
Large oral doses of nystatin have occasionally produced diarrhea, gastrointestinal distress, and possible irritation of the stomach that may result in nausea and vomiting.
NYSTATIN DOSAGE AND ADMINISTRATION
General
Adults and older children: Add approximately 500,000 units of Nystatin USP to about 1/2 cup of water and stir well. 500,000 units of Nystatin USP is equivalent to the recommended dose for adults and children of Nystatin Oral Suspension (4 to 6 mL, or 400,000 to 600,000 units). This product contains no preservatives and therefore should be used immediately after mixing and should not be stored. It is designed for extemporaneous preparation of a single dose at a time.
Infections of the oral cavity caused by (Monilia)
Infants: 200,000 units four times daily.
Children and adults: 400,000 to 600,000 units four times daily (one-half dose in each side of mouth).
NOTE: Limited clinical studies in premature and low birth-weight infants indicate that 100,000 units four times daily is effective.
Local treatment should be continued at least 48 hours after perioral symptoms have disappeared and cultures returned to normal.
It is recommended that the drug be retained in the mouth as long as possible before swallowing.
Intestinal candidiasis (moniliasis)
Usual dosage: 500,000 to 1 million units three times daily. Treatment should generally be continued for at least 48 hours after clinical cure to prevent relapse.
HOW SUPPLIED
Nystatin USP is supplied in containers of 50 million, 150 million, 500 million, and 2 billion units.
| Product Code (NDC) | Size (Units) | Approx. Weight (grams) |
|---|---|---|
| 0574-0404-05 | 50 million | 8.3 - 10 |
| 0574-0404-15 | 150 million | 25 - 30 |
| 0574-0404-50 | 500 million | 83 - 100 |
| 0574-0404-02 | 2 billion | 333 - 400 |
Storage: Store in a refrigerator, 2°-8°C (36°-46°F). Protect from light. Dispense in a tight, light-resistant container.
NOTE: The potency of this product cannot be assured for longer than 90 days after the container is first opened.
Packaged by:
Paddock Laboratories, Inc.
Minneapolis, MN 55427
(08-05)
NystatinNystatin POWDER, FOR SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||