Omidria
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGEOMIDRIA is an alpha 1-adrenergic receptor agonist and nonselective cyclooxygenase inhibitor indicated for: Maintaining pupil size by preventing intraoperative miosis (1) Reducing postoperative pain (1)OMIDRIA is added to an irrigation solution used during cataract surgery or intraocular lens replacement.DOSAGE AND ADMINISTRATIONOMIDRIA must be diluted prior to use. For administration to patients undergoing cataract surgery or intraocular lens replacement, 4 mL of OMIDRIA is diluted in 500 mL of ophthalmic irrigating solution. Irrigation solution is to be used as needed for the surgical procedure. (2)DOSAGE FORMS AND STRENGTHSOMIDRIA is a sterile solution concentrate containing 1% w/v of phenylephrine and 0.3% w/v ketorolac in a single-patient-use vial. (3)CONTRAINDICATIONSNone. (4)WARNINGS AND PRECAUTIONSSystemic exposure of phenylephrine may cause elevations in blood pressure. (5.1)Side EffectsThe most common reported adverse reactions at 2-24% are eye irritation, posterior capsule opacification, increased intraocular pressure, and anterior chamber inflammation. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Omeros Corporation at 1-844-OMEROS1 or www.omidria.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 OMIDRIA INDICATIONS AND USAGE
- 2 OMIDRIA DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 OMIDRIA CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 OMIDRIA ADVERSE REACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 OMIDRIA DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Omidria™ is added to an ophthalmic irrigation solution used during cataract surgery or intraocular lens replacement and is indicated for maintaining pupil size by preventing intraoperative miosis and reducing postoperative ocular pain.
2 DOSAGE AND ADMINISTRATION
Omidria must be diluted prior to intraocular use. For administration to patients undergoing cataract surgery or intraocular lens replacement, 4 mL of Omidria is diluted in 500 mL of ophthalmic irrigation solution. Irrigation solution is to be used as needed for the surgical procedure.
The storage period for the diluted product is not more than 4 hours at room temperature or 24 hours under refrigerated conditions.
Do not use if the solution is colored or cloudy, or if it contains particulate matter.
3 DOSAGE FORMS AND STRENGTHS
Omidria is a sterile solution concentrate containing 10.16 mg/mL (1% w/v) of phenylephrine and 2.88 mg/mL (0.3% w/v) of ketorolac in a single-patient-use vial.
4 CONTRAINDICATIONS
Omidria is contraindicated in patients with a known hypersensitivity to any of its ingredients.
5 WARNINGS AND PRECAUTIONS
5.1 Elevated Blood Pressure
Systemic exposure of phenylephrine can cause elevations in blood pressure.
5.2 Cross-Sensitivity or Hypersensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other non-steroidal anti-inflammatories (NSAIDs). There have been reports of bronchospasm or exacerbation of asthma associated with the use of ketorolac in patients who either have a known hypersensitivity to aspirin/NSAIDs or a past medical history of asthma. Therefore, use Omidria with caution in individuals who have previously exhibited sensitivities to these drugs.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Table 1 shows frequently reported ocular adverse reactions with an incidence of ≥ 2% of subjects as seen in the combined clinical trial results from three randomized, placebo-controlled studies.
| MedDRA Preferred Term |
Placebo (N=462) |
Omidria (N=459) |
| n (%) | n (%) | |
| Ocular Events | ||
| Anterior Chamber Inflammation | 102 (22%) | 111 (24%) |
| Intraocular Pressure Increased | 15 (3%) | 20 (4%) |
| Posterior Capsule Opacification | 16 (4%) | 18 (4%) |
| Eye Irritation | 6 (1%) | 9 (2%) |
| Foreign Body Sensation in Eyes | 11 (2%) | 8 (2%) |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C
Animal reproduction studies have not been conducted with Omidria or phenylephrine. It is also not known whether Omidria can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Omidria should be used in pregnant women only if clearly needed.
Ketorolac, administered during organogenesis, was not teratogenic in rabbits or rats at oral doses of 3.6 mg/kg/day and 10 mg/kg/day, respectively. These doses produced systemic exposure that is 1150 times and 4960 times the plasma exposure (based on Cmax) at the recommended human ophthalmic dose (RHOD), respectively. When administered to rats after Day 17 of gestation at oral doses up to 1.5 mg/kg/day (740 times the plasma exposure at the RHOD), ketorolac produced dystocia and increased pup mortality.
Clinical Considerations:
Premature closure of the ductus arteriosus in the fetus has occurred with third trimester use of oral and injectable NSAIDs. Detectable ketorolac plasma concentrations are available following ocular Omidria administration [see Clinical Pharmacology (12.3)]. The use of Omidria during late pregnancy should be avoided.
8.3 Nursing Mothers
Because many drugs are excreted in human milk, caution should be exercised when Omidria is administered to nursing women.
8.4 Pediatric Use
Safety and effectiveness of Omidria in pediatric patients below the age of 18 years have not been established.
8.5 Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and adult patients.
10 OVERDOSAGE
Systemic overdosage of phenylephrine may cause a rise in blood pressure. It may also cause headache, anxiety, nausea, vomiting, and ventricular arrhythmias. Supportive care is recommended.
11 DESCRIPTION
Omidria is a sterile aqueous solution concentrate containing the α1-adrenergic receptor agonist phenylephrine HCl and the nonsteroidal anti-inflammatory ketorolac tromethamine.
The descriptions and structural formulae are:
|
Common Name: |
phenylephrine hydrochloride |
|
Chemical Name: |
(‑)‑m‑Hydroxy‑α‑[(methylamino)methyl]benzyl |
|
Molecular Formula: |
C9H13NO2 · HCl |
|
Molecular Weight: |
203.67 g/mole |
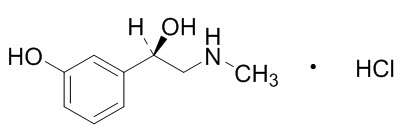
Omidria is a clear, colorless, sterile solution concentrate with a pH of approximately 6.3.
Each vial of Omidria contains:
Actives: phenylephrine hydrochloride 12.4 mg/mL equivalent to 10.16 mg/mL of phenylephrine and ketorolac tromethamine 4.24 mg/mL equivalent to 2.88 mg/mL of ketorolac.
Inactives: citric acid monohydrate; sodium citrate dihydrate; water for injection; may include sodium hydroxide and/or hydrochloric acid for pH adjustment.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The two active pharmaceutical ingredients (API) in Omidria, phenylephrine and ketorolac, act to maintain pupil size by preventing intraoperative miosis, and reducing postoperative pain.
Phenylephrine is an α1-adrenergic receptor agonist and, in the eye, acts as a mydriatic agent by contracting the radial muscle of the iris. Ketorolac is a nonsteroidal anti-inflammatory that inhibits both cyclooxygenase enzymes (COX-1 and COX-2), resulting in a decrease in tissue concentrations of prostaglandins to reduce pain due to surgical trauma. Ketorolac, by inhibiting prostaglandin synthesis secondary to ocular surgical insult or direct mechanical stimulation of the iris, also prevents surgically induced miosis.
12.3 Pharmacokinetics
In a pharmacokinetic study evaluating Omidria, systemic exposure to both phenylephrine and ketorolac was low or undetectable.
A single-dose of Omidria as part of the irrigation solution was administered in 14 patients during lens replacement surgery. The volume of irrigation solution used during surgery ranged between 150 mL to 300 mL (median 212.5 mL). Detectable phenylephrine plasma concentrations were observed in one of 14 subjects (range 1.2 to 1.4 ng/mL) during the first two hours after the initiation of Omidria administration. The observed phenylephrine plasma concentrations could not be distinguished from the preoperative administration of phenylephrine 2.5% ophthalmic solution prior to exposure to Omidria.
Ketorolac plasma concentrations were detected in 10 of 14 subjects (range 1.0 to 4.2 ng/mL) during the first 8 hours after the initiation of Omidria administration. The maximum ketorolac concentration was 15 ng/mL at 24 hours after the initiation of Omidria administration, which may have been due to application of postoperative ketorolac ophthalmic solution.
14 CLINICAL STUDIES
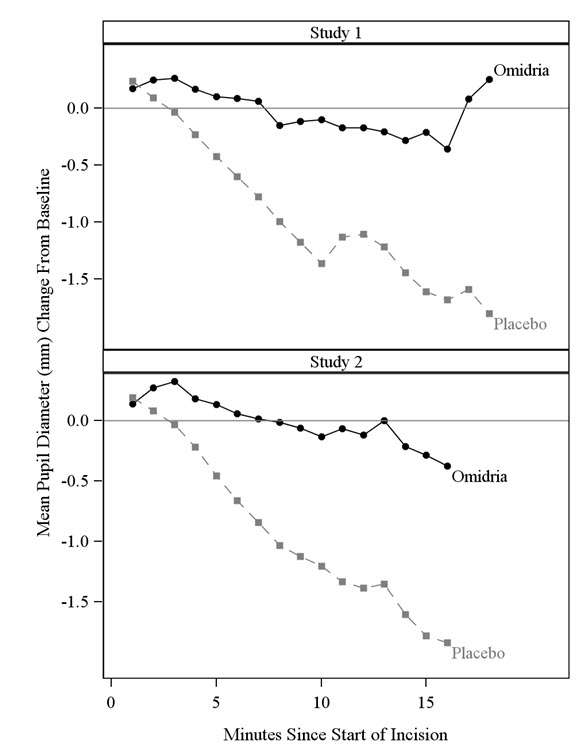
The efficacy and safety of Omidria were evaluated in two Phase 3, randomized, multicenter, double-masked, placebo-controlled clinical trials in 808 adult subjects undergoing cataract surgery or intraocular lens replacement.
Subjects were randomized to either Omidria or placebo. Subjects were treated with preoperative topical mydriatic and anesthetic agents. Pupil diameter was measured throughout the surgical procedure. Postoperative pain was evaluated by self-administered 0-100 mm visual analog scales (VAS).
Mydriasis was maintained in the Omidria-treated groups while the placebo-treated groups experienced progressive constriction.
During the 10-12 hours postoperatively, 26% of Omidria-treated subjects reported no pain (VAS = 0 at all timepoints) while 17% of placebo-treated subjects reported no pain (p < 0.01).
16 HOW SUPPLIED/STORAGE AND HANDLING
Omidria is supplied as a sterile solution concentrate in a clear, 5-mL glass, single-patient-use vial containing 4 mL of sterile solution.
Omidria is supplied in a multi-pack containing:
4 single-patient-use vials: NDC 62225-600-04 or
10 single-patient-use vials: NDC 62225-600-10
Storage: Store at 20˚ to 25˚C (68˚ to 77˚F). Protect from light.
17 PATIENT COUNSELING INFORMATION
Inform patients that they may experience sensitivity to light.
Omeros Corporation
201 Elliott Avenue West
Seattle, WA 98119
© Omeros 2014
US Patents 8,173,707 and 8,586,633; additional patents pending.
Revised: 05/2014
PRINCIPAL DISPLAY PANEL
NDC 62225-600-10
OMIDRIA
(phenylephrine and ketorolac injection) 1% / 0.3%
For Intraocular use.
Must Be Diluted/
Quantity: 10
Rx Only

Omidriaphenylephrine and ketorolac INJECTION, SOLUTION, CONCENTRATE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||