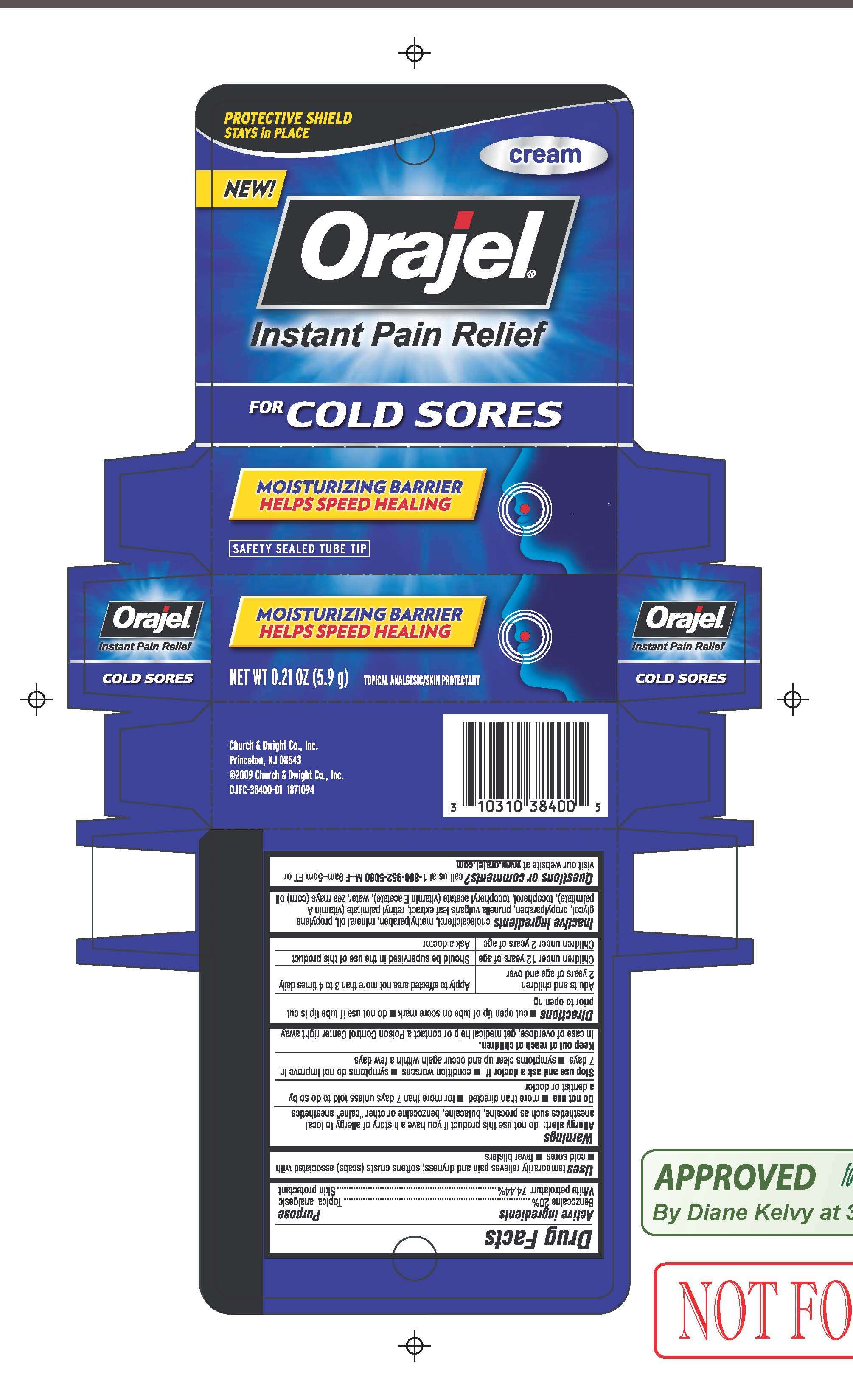
Orajel for Cold Sores
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Benzocaine 20% Oral pain reliever
White petrolatum 74.44% Skin protectant
Purpose
Uses temporarily relieves pain and dryness; softens crusts (scabs) associated with cold sores, fever blisters
Warnings
Allergy Alert
do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Do not use
more than is directed
for more than 7 days unless told to do so by a dentist or doctor
Stop use and ask a doctor if
condition worsens
symptoms do not improve in 7 days
symptoms clear up and occur again within a few days
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away
Directions cut open tip of tube on score mark; do not use if tube tip is cut prior to opening
Adults and children 2 years of age and over Apply to affected area not more than 3 to 4 times daily
Children under 12 years of age Should be supervised in the use of this product
Children under 2 years of age Ask a doctor
Inactive ingredients
cholecalciferol, methylparaben, mineral oil, propylene glycol, propylparaben, prunella vulgaris leaf extract, retinyl palmitate (vitamin A palmitate), tocopherol, tocopheryl acetate (vitamin E acetate), water, zea mays (corn) oil
Questions or comments? call us at 1-800-952-5080 M-F 9am-5pm or visit our website at www.orajel.com
Orajel for Cold SoresBenzocaine CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||