Orajel Instant Pain Relief for Toothache
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Do not useStop use and ask a doctor if
Keep out of reach of children:
in case of overdose, get medical help or contact a Poison Control Center right away
Directions
Directions cut open tip of tube on score mark
Adults and children 2 years of age and over Apply a small amount of product to the cavity and around gum surrounding the teeth.
Use up to 4 times daily or as directed by a dentist or doctor
Other information
Inactive ingredients
Inactive ingredients ammonium glycyrrhizate, flavor, polyethylene glycol, sodium saccharin, sorbic acid
Questions or comments? call us at 1-800-952-5080 M-F 9am-5pm ET or visit our website at www.orajel.com
Orajel gel
Instant Pain Relief
for TOOTHACHE
MAXIMUM STRENGTH
NE WT 0.42 OZ (11.9 g) ORAL PAIN RELIEVER BENZOCAINE 20%
carton image
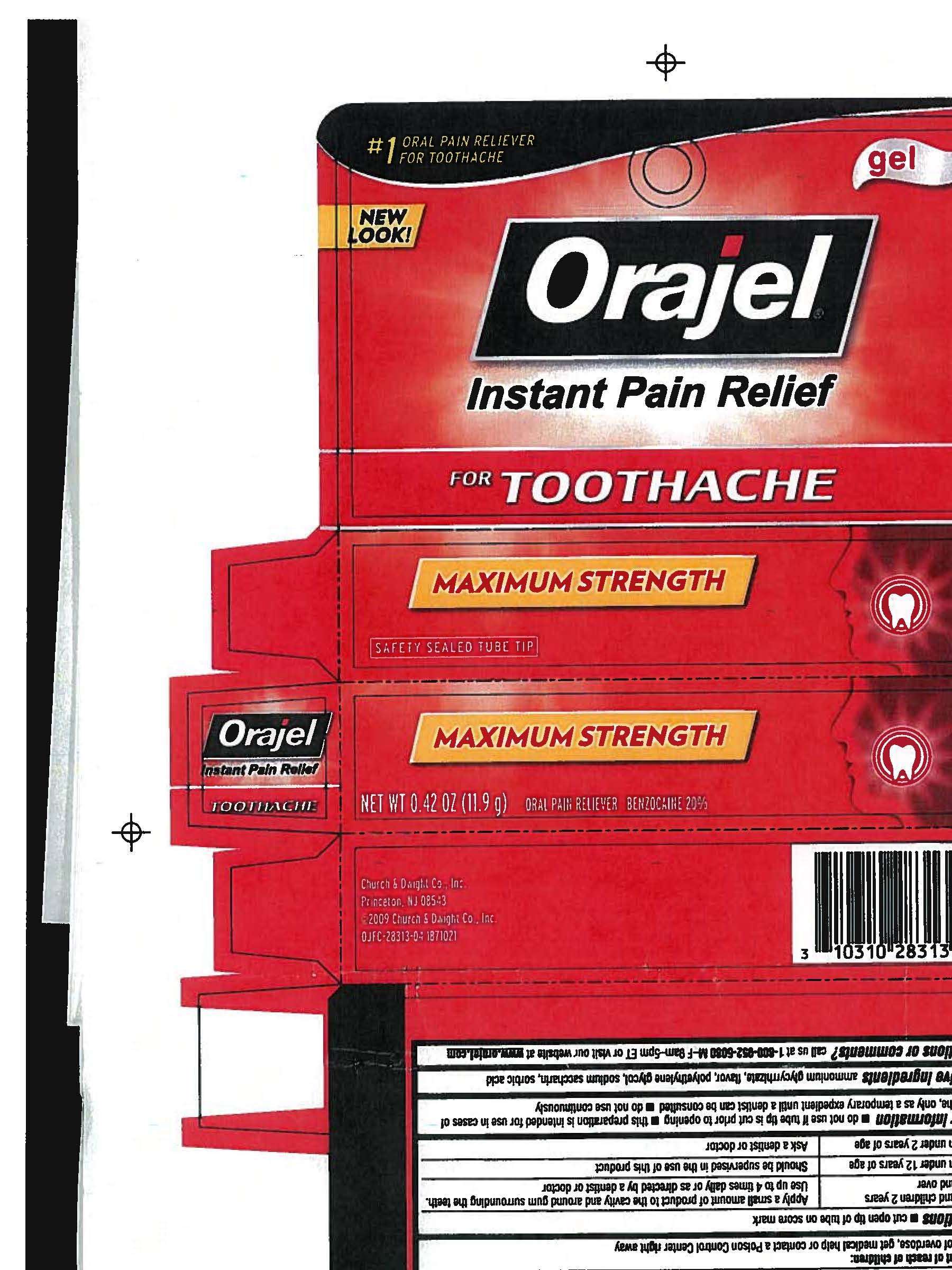
Orajel Instant Pain Relief for ToothacheOral Pain Reliever GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||