Oral B Instant Pain Relief
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Oral B Instant Pain Relief Uses
- Warnings
- When using this product
- Stop use and ask a doctor if:
- Directions:
- Oral B Instant Pain Relief Other information:
- Inactive Ingredients
- Oral B Pain gel label
- Oral B pain gel blister
- Oral B shipper label
FULL PRESCRIBING INFORMATION
Active Ingredient
Benzocaine 20%
Purpose
Oral anesthetic
Oral B Instant Pain Relief Uses
Temporarily relieves pain associated with the following mouth and gum irritations:
- toothache
- sore gums
- canker sores
- braces
- minor dental procedures
- dentures
Warnings
Allergy Alert: do not use this pr9oduct if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other :caine" anesthetics.
When using this product
- Avoid contact with eyes
- Do not exceed recommended dosage
- Do not use for more than 7 days unless directed by a doctor/dentist
Stop use and ask a doctor if:
- sore mouth symptoms do not improve in 7 days
- Irritation, pain or redness persist or worsens
- Swelling, pain or fever develops
Directions:
- Remove cap-, squeeze gel onto applicator
- Adults and children 2 years of age and older, apply to the affected area 4 times per day or as directed by a doctor/dentist
- Children under 2 years of age; consult a doctor/dentist
- Remove Dentures
- Apply thin layer to affected area
- Do not reinsert dental work until irritation is relieved
- Rinse mouth well before reinserting dentures
Other information:
- Store between 200 to 250 C (680 to 770 F) Do not refrigerate
- Do not purcahse if package has been opened
Inactive Ingredients
Benzyl alcohol, carbomer 974P, FD and C green 5, Flavor, glycerin, Methylparaben, Polyethylene glycol, PEG 400, PEG 600, Sodium Saccharin
Oral B Pain gel label
MM27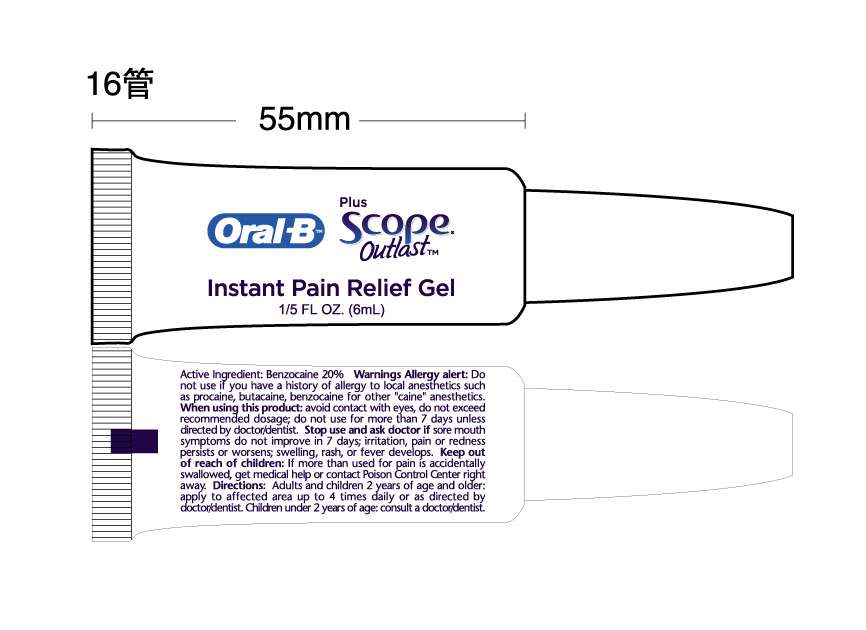
Oral B pain gel blister
MM28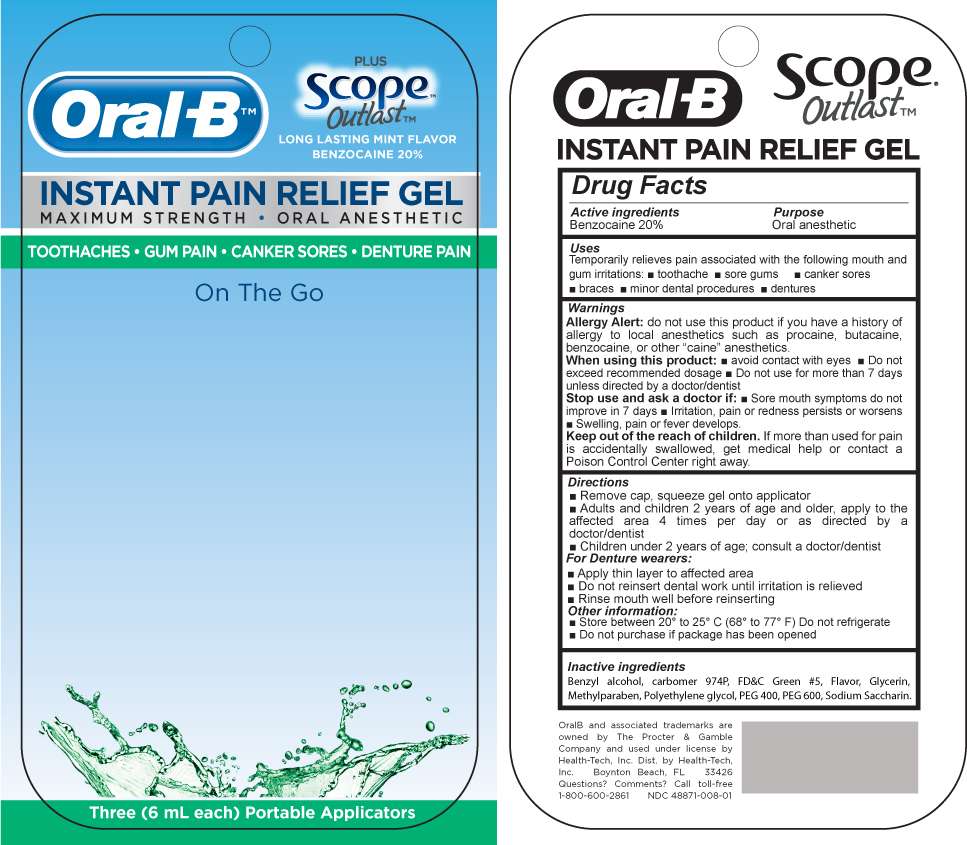
Oral B shipper label
MM29
Oral B Instant Pain Reliefbenzocaine GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!