Orphenadrine Citrate
Lake Erie Medical DBA Quality Care Products LLC
Orphenadrine Citrate Extended-Release Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- ORPHENADRINE CITRATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- ORPHENADRINE CITRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PREGNANCY
- PEDIATRIC USE
- ORPHENADRINE CITRATE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- ORPHENADRINE CITRATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Image of Label
FULL PRESCRIBING INFORMATION
Rx only
ORPHENADRINE CITRATE DESCRIPTION
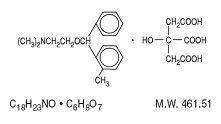
Orphenadrine citrate is the citrate salt of orphenadrine: (2-dimethyl-aminoethyl 2-methylbenzhydryl ether citrate). It occurs as a white, crystalline powder having a bitter taste. It is practically odorless; sparingly soluble in water, slightly soluble in alcohol and has a molecular weight of 461.51. The molecular formula C18H23NO • C6H8O7 is represented by the following structural formula:
Each orphenadrine citrate extended-release tablet contains 100 mg orphenadrine citrate. Orphenadrine citrate extended-release tablets also contain: calcium stearate, ethylcellulose and lactose monohydrate.
CLINICAL PHARMACOLOGY
The mode of therapeutic action has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate extended-release tablets do not directly relax tense skeletal muscles in man. Orphenadrine citrate extended-release tablets also possess anti-cholinergic actions.
INDICATIONS
Orphenadrine citrate is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculo skeletal conditions. The mode of action of the drug has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate does not directly relax tense skeletal muscles in man.
ORPHENADRINE CITRATE CONTRAINDICATIONS
Contraindicated in patients with glaucoma, pyloric or duodenal obstruction, stenosing peptic ulcers, prostatic hypertrophy or obstruction of the bladder neck, cardio-spasm (megaesophagus) and myasthenia gravis.
Contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
WARNINGS
Some patients may experience transient episodes of light-headedness, dizziness or syncope. Orphenadrine citrate extended-release tablets may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore be cautioned accordingly.
PRECAUTIONS
Confusion, anxiety and tremors have been reported in few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
Orphenadrine citrate extended-release tablets should be used with caution in patients with tachycardia, cardiac decompensation, coronary insufficiency, cardiac arrhythmias.
Safety of continuous long-term therapy with orphenadrine citrate extended-release tablets has not been established. Therefore, if orphenadrine citrate extended-release tablets are prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
PREGNANCY
Pregnancy Category C
Animal reproduction studies have not been conducted with orphenadrine citrate extended-release tablets. It is also not known whether orphenadrine citrate extended-release tablets can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Orphenadrine citrate extended-release tablets should be given to a pregnant woman only if clearly needed.
PEDIATRIC USE
Safety and effectiveness in pediatric patients have not been established.
ORPHENADRINE CITRATE ADVERSE REACTIONS
Adverse reactions of orphenadrine citrate extended-release tablets are mainly due to the mild anti-cholinergic action of orphenadrine citrate extended-release tablets and are usually associated with higher dosage. Dryness of the mouth is usually the first adverse effect to appear. When the daily dose is increased, possible adverse effects include tachycardia, palpitation, urinary hesitancy or retention, blurred vision, dilatation of pupils, increased ocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, hypersensitivity reactions, pruritus, hallucinations, agitation, tremor, gastric irritation and rarely urticaria and other dermatoses. Infrequently, an elderly patient may experience some degree of mental confusion. These adverse reactions can usually be eliminated by reduction in dosage. Very rare cases of aplastic anemia associated with the use of orphenadrine tablets have been reported. No causal relationship has been established.
DRUG ABUSE AND DEPENDENCE
Orphenadrine citrate extended-release tablets have been chronically abused for their euphoric effects.[1] The mood elevating effects may occur at therapeutic doses of orphenadrine.[2]
OVERDOSAGE
Orphenadrine citrate extended-release tablets are toxic when overdosed and typically induces anti-cholinergic effects.[3] In a review of orphenadrine toxicity, the minimum lethal dose was found to be 2 to 3 grams for adults; however, the range of toxicity is variable and unpredictable.[4] Treatment for orphenadrine citrate extended-release tablets overdose is evacuation of stomach contents (when necessary), charcoal at repeated doses, intensive monitoring and appropriate supportive treatment of any emergent anti-cholinergic effects.[5]
ORPHENADRINE CITRATE DOSAGE AND ADMINISTRATION
Adults-Two tablets per day; one in the morning and one in the evening.
HOW SUPPLIED
Orphenadrine Citrate Extended-Release Tablets, 100 mg, white, round-shaped tablets debossed “E” over “22” on one side and plain on the other side and supplied as:
Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
Dispense in tight, light-resistant containers as defined in the USP, with a child-resistant closure as required.
KEEP TIGHTLY CLOSED.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Sandoz Inc.
Princeton, NJ 08540
OS8870
Rev. 09/09
MF0022REV09/09
MG #14534
Image of Label

Orphenadrine CitrateOrphenadrine Citrate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||