Oxcarbazepine
FULL PRESCRIBING INFORMATION: CONTENTS*
- OXCARBAZEPINE DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- OXCARBAZEPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- OXCARBAZEPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
OXCARBAZEPINE DESCRIPTION
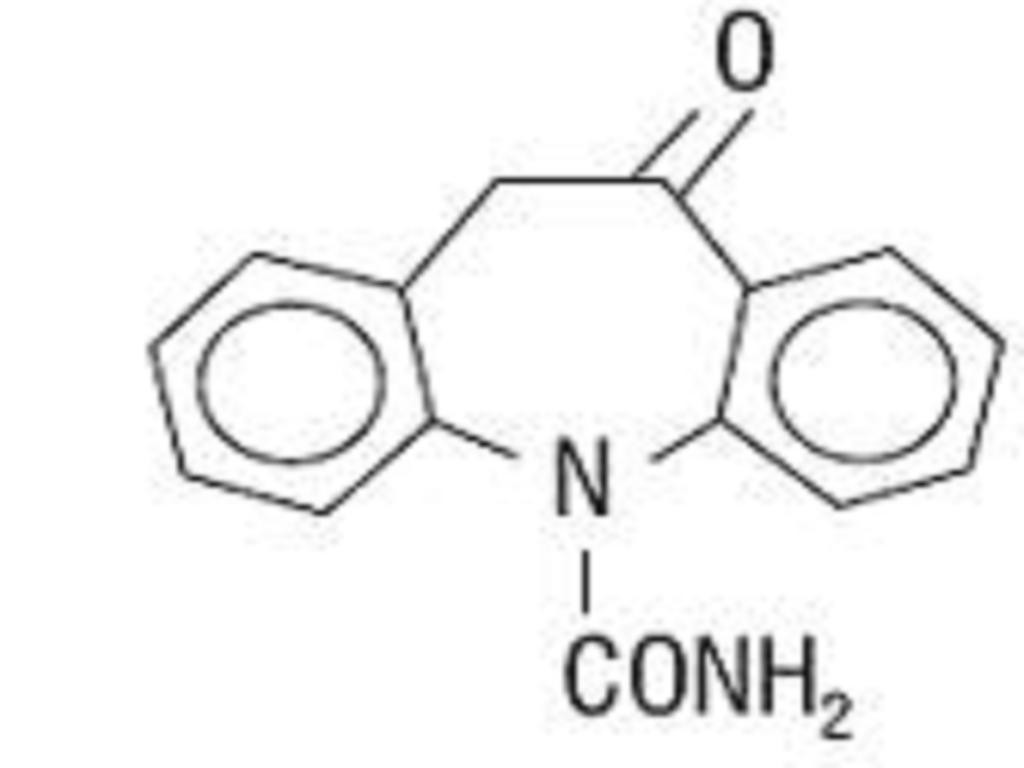
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
Mechanism of ActionMetabolism and Excretion subsection
PHARMACODYNAMICS
PharmacodynamicsPHARMACOKINETICS
PharmacokineticsDistribution
Metabolism and Excretion
Special Populations
Hepatic Impairment
Renal Impairment
PRECAUTIONSDOSAGE AND ADMINISTRATION
Pediatric Use
Geriatric Use
Gender
Race
CLINICAL STUDIES
Oxcarbazepine Monotherapy Trials
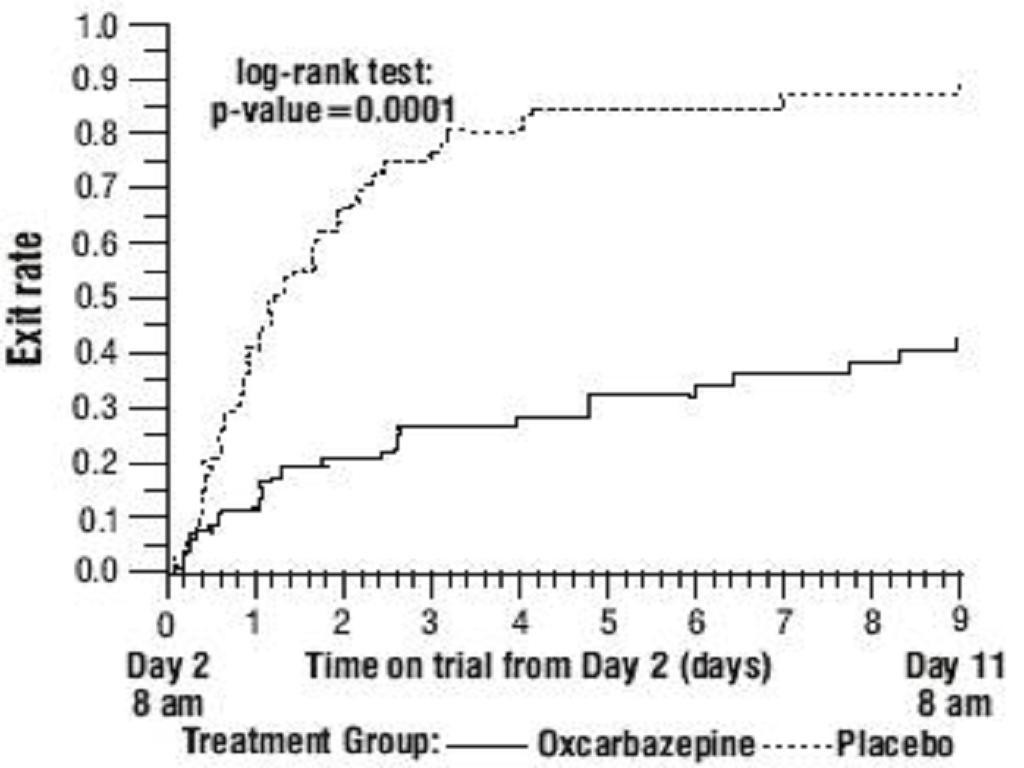
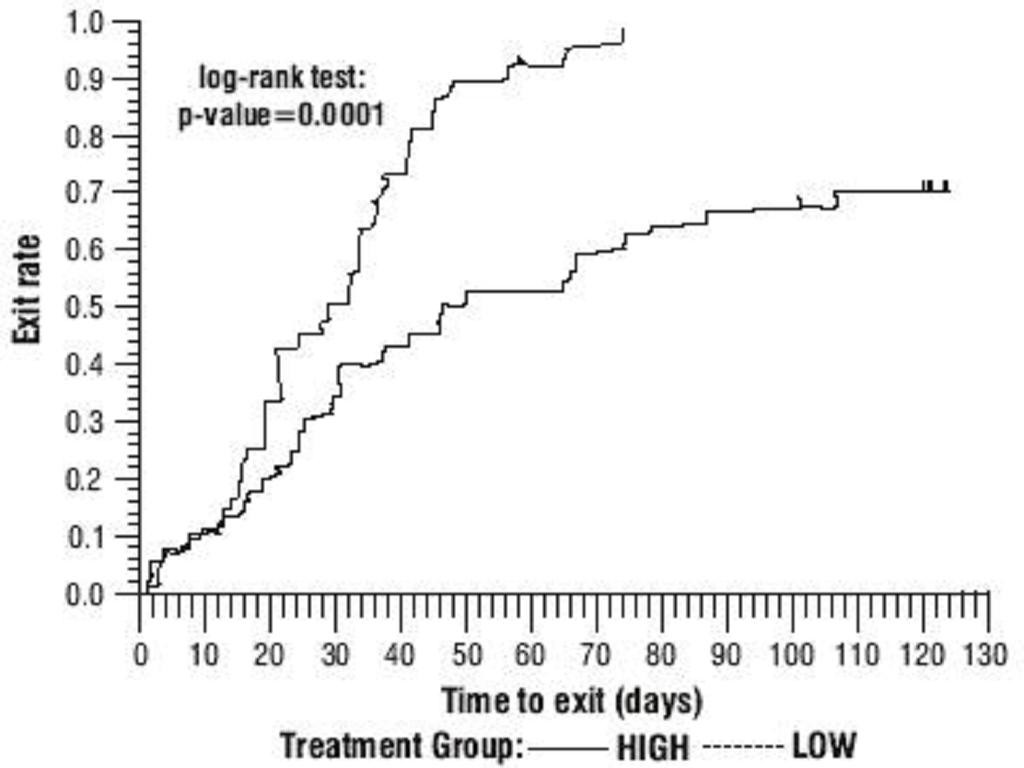
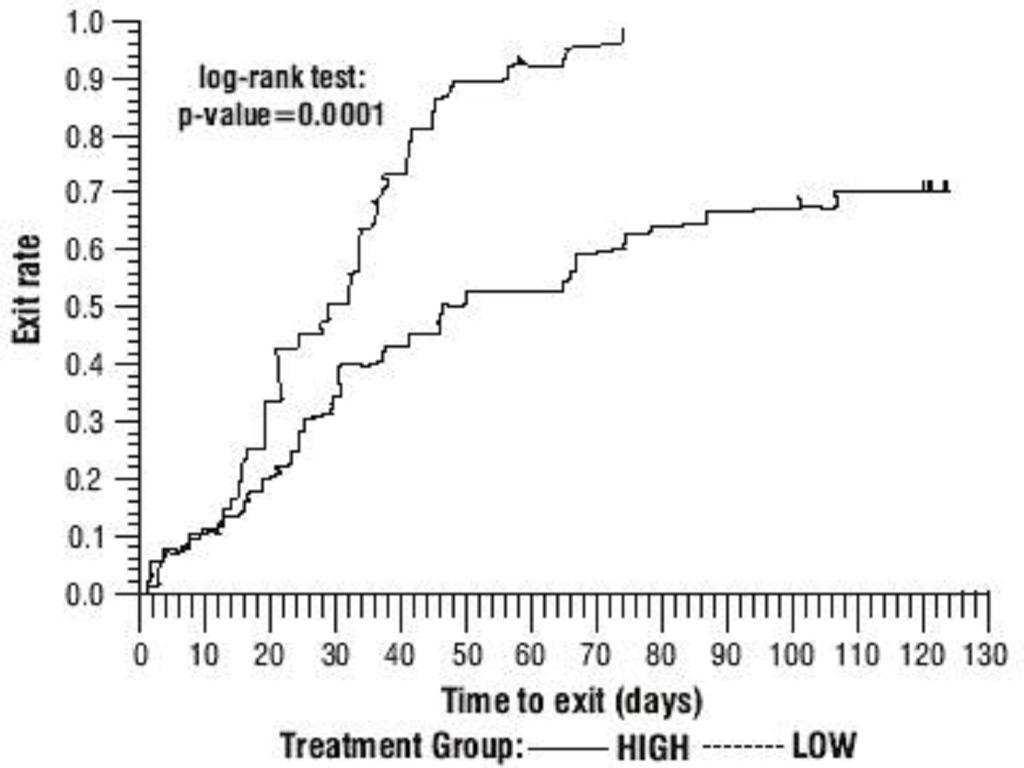
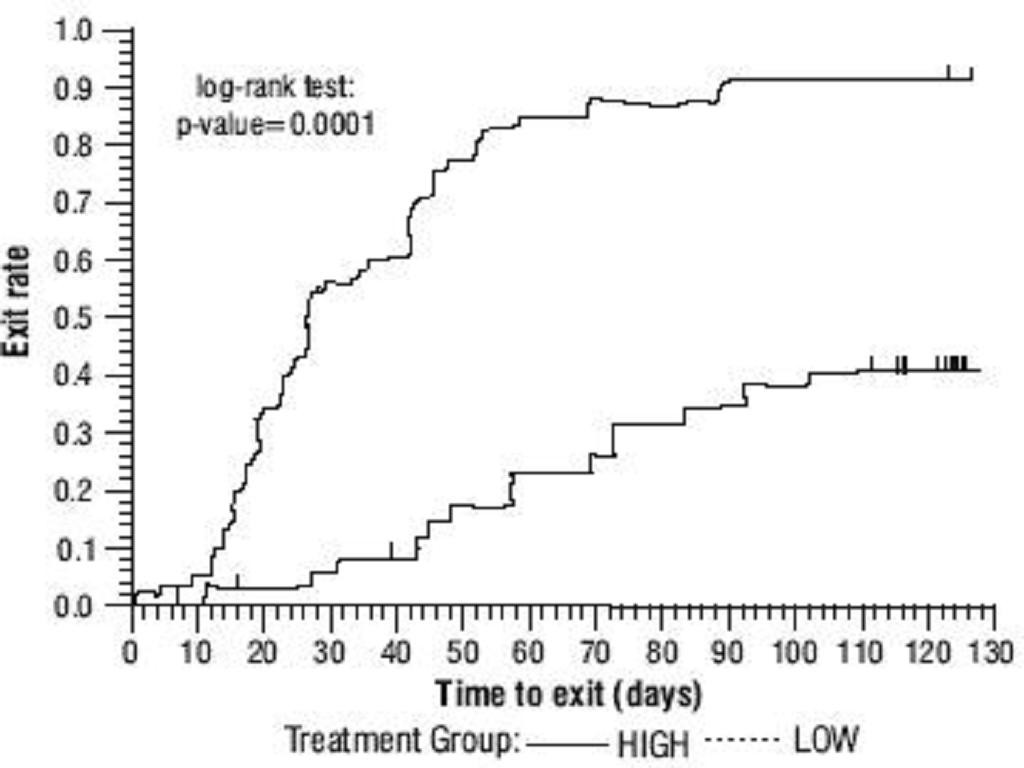
Oxcarbazepine Adjunctive Therapy Trials
Table 1ADVERSE REACTIONS
TrialTreatment GroupBaseline MedianMedian %NSeizure Rate*Reduction
INDICATIONS & USAGE
OXCARBAZEPINE CONTRAINDICATIONS
WARNINGS
HyponatremiaAnaphylactic Reactions and Angioedema
WARNINGS, Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
WARNINGS, Anaphylactic Reactions and AngioedemaPRECAUTIONS, Multi-Organ Hypersensitivity
Serious Dermatological Reactions
Suicidal Behavior and Ideation
Table 2
Withdrawal of AEDs
PRECAUTIONS
Cognitive/Neuropsychiatric Adverse EventsAdult Patients
Pediatric Patients
Multi-Organ Hypersensitivity
WARNINGS, Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
INFORMATION FOR PATIENTS
WARNINGS, Anaphylactic Reactions and AngioedemaWARNINGS, Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
WARNINGS, Serious Dermatological Reactions
PRECAUTIONS, Multi-organ Hypersensitivity
Drug Interactions
PRECAUTIONS, Pregnancy Category C
LABORATORY TESTS
WARNINGSDRUG INTERACTIONS
Antiepileptic Drugs
Table 3
Hormonal Contraceptives
Drug Interactions
Calcium Antagonists
Other Drug Interactions
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis/Mutagenesis/Impairment of FertilityPREGNANCY
Category CLABOR & DELIVERY
Labor and DeliveryNURSING MOTHERS
Nursing MothersPatients with Renal Impairment
CLINICAL PHARMACOLOGY, Pharmacokinetics
PEDIATRIC USE
Pediatric UseADVERSE REACTIONS
GERIATRIC USE
Geriatric UseOXCARBAZEPINE ADVERSE REACTIONS
Most Common Adverse Events in All Clinical StudiesTable 4Table 5
Oxcarbazepine Dosage (mg/day)Body System/OXC 600OXC 1200OXC 2400PlaceboAdverse EventN = 163N = 171N = 126N = 166%%%%Body as a WholeCardiovascular SystemDigestive SystemMetabolic and Nutritional DisordersMusculoskeletal SystemNervous SystemRespiratory SystemSkin and AppendagesSpecial Senses
Oxcarbazepine Dosage (mg/day)Body System/2400300Adverse EventN = 86N = 86%%Body as a WholeDigestive SystemHemic and Lymphatic SystemInfections and InfestationsMetabolic and Nutritional DisordersNervous SystemRespiratory SystemSkin and AppendagesSpecial SensesUrogenital and Reproductive SystemTable 6
Body System/OxcarbazepinePlaceboAdverse EventN = 55N = 49%%Body as a WholeDigestive SystemMusculoskeletal SystemNervous SystemRespiratory SystemSkin and AppendagesSpecial SensesTable 7
Body System/OxcarbazepinePlaceboAdverse EventN = 171N = 139%%Body as a WholeDigestive SystemNervous SystemRespiratory SystemSkin and AppendagesSpecial Senses
Other Events Observed in Association with the Administration of Oxcarbazepine
Post-Marketing and Other Experience
PRECAUTIONS, Multi-Organ Hypersensitivity
WARNINGS, Anaphylactic Reactions and Angioedema
WARNINGS, Serious Dermatological Reactions
DRUG ABUSE AND DEPENDENCE
AbuseDependence
OVERDOSAGE
Human Overdose ExperienceTreatment and Management
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGY, Pharmacokinetics
Adults
Adjunctive Therapy
PRECAUTIONS, Drug Interactions
Conversion to Monotherapy
Initiation of Monotherapy
Pediatric Patients
Adjunctive Therapy (Aged 2 to 16 Years)
CLINICAL PHARMACOLOGY
Conversion to Monotherapy (Aged 4 to 16 Years)
Initiation of Monotherapy (Aged 4 to 16 Years)
FromToWeightDoseDosein kg(mg/day)(mg/day)
Patients with Hepatic Impairment
CLINICAL PHARMACOLOGYPharmacokineticsSpecial Populations
Patients with Renal Impairment
CLINICAL PHARMACOLOGYPharmacokineticsSpecial Populations
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

OxcarbazepineOxcarbazepine TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!