OXYGEN
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
OXYGEN COMPRESSED USP LABEL
OXYGEN COMPRESSED USP UN1072 C.A.S. NO. 7782-44-7 OXYGEN-2 WARNING: HIGH PRESSURE OXIDIZING GAS. VIGOROUSLY ACCELERATES COMBUSTION. NO SMOKING IN THE PRESENCE OF OXYGEN OR A FIRE MAY RESULT. KEEP OIL AND GREASE AWAY. OPEN VALVE SLOWLY. STORE AND USE WITH ADEQUATE VENTILATION. USE ONLY WITH EQUIPMENT CLEANED FOR OXYGEN SERVICE AND RATED FOR CYLINDER PRESSURE. USE A BACK FLOW PREVENTATIVE DEVICE IN THE PIPING. CLOSE VALVE AFTER EACH USE AND WHEN EMPTY. CYLINDER TEMPERATURE SHOULD NOT EXCEED 52 C (125 F) USE IN ACCORDANCE WITH THE MATERIAL SAFETY DATA SHEET (MSDS) DO NOT REMOVE THIS PRODUCT LABEL.
WARNING: FOR EMERGENCY USE ONLY WHEN ADMINISTERED BY PROPERLY TRAINED PERSONNEL FOR OXYGEN DEFICIENCY AND RESUSCITATION. FOR ALL OTHER MEDICAL APPLICATIONS Rx ONLY. UNINTERRUPTED USE OF HIGH CONCENTRATIONS OF OXYGEN OVER A LONG DURATION WITHOUT MONITORING ITS EFFECT ON OXYGEN CONTENT OF ARTERIAL BLOOD MAY BE HARMFUL. USE ONLY WITH PRESSURE REDUCING EQUIPMENT AND APPARATUS DESIGNED FOR OXYGEN. DO NOT ATTEMPT TO USE ON PATIENTS WHO HAVE STOPPED BREATHING UNLESS USED IN CONJUNCTION WITH RESUSCITATIVE EQUIPMENT. PRODUCED BY AIR LIQUEFACTION.

OXYGEN REFRIGERATED LIQUID USP LABEL
OXYGEN REFRIGERATED LIQUID USP UN1073 ALWAYS KEEP CONTAINER IN UPRIGHT POSITION. EXTREMELY COLD LIQUID AND GAS UNDER PRESSURE. VIGOROUSLY ACCELERATES COMBUSTION. MAY EXPLODE ON IGNITION OR IMPACT. CAN CAUSE SEVERE FROSTBITE. KEEP OIL, GREASE AND COMBUSTIBLES AWAY. USE ONLY WITH EQUIPMENT CLEANED FOR OXYGEN SERVICE. STORE AND USE WITH ADEQUATE VENTILATION. DO NOT GET LIQUID IN EYES, ON SKIN OR CLOTHING. FOR LIQUID WITHDRAWAL, WEAR FACE SHIELD AND GLOVES. DO NOT DROP. USE HAND TRUCK FOR CONTAINER MOVEMENT. AVOID SPILLS. DO NOT WALK OR ROLL EQUIPMENT OVER SPILLS CLOSE VALVE AFTER EACH USE AND WHEN EMPTY.
FIRST AID: IN CASE OF FROSTBITE OBTAIN MEDICAL TREATMENT IMMEDIATELY.
KEEP OUT OF THE REACH OF CHILDREN. DO NOT USE OR STORE NEAR HEAT OR OPEN FLAME. FEDERAL LAW REQUIRES THAT THIS CONTAINER BE REFILLED WITH LIQUID OXYGEN USP ONLY BE ESTABLISHED REGISTERED AS A DRUG PRODUCER IN ACCORDANCE WITH THE FEDERAL FOOD DRUG AND COSMETIC ACT. PRODUCED BY AIR LIQUEFACTION. DO NOT REMOVE THIS PRODUCT LABEL.
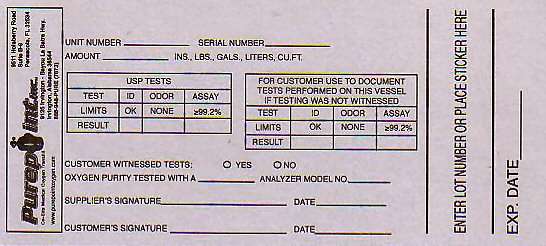
LOT NUMBER_____________ SERIAL NUMBER__________ AMOUNT__________INS, LBS., GALS., LITERS, CU. FT.
USP TESTS
TEST ID ODOR ASSAY
LIMITS OK NONE LESS THAN 99.2%
RESULT
FOR CUSTOMER TO USE TO DOCUMENT TESTS PERFORMED ON THIS VESSEL
METHOD OF ANALYSIS FOR ASSAY IS THE PARAMAGNETIC OXYGEN ANALYZER MANUFACTURER__________ MODEL______________ CUSTOMER WITNESSED TESTS YES NO SUPPLIERS SIGNATURE__________ DATE____________ CUSTOMERS SIGNATURE_______________ DATE________________
LIQUID MEDICAL GASES LIQUID OXYGEN DELIVERY TAG FILLED AND DISTRIBUTED BY____________ THIS VESSEL CONTAINS OXYGEN USP (SEE REVERSE SIDE FOR TEST RESULTS) OXYGEN PRODUCED BY THE AIR LIQUEFACTION PROCESS

OXYGENOXYGEN GAS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||