OXYGEN
FULL PRESCRIBING INFORMATION: CONTENTS*
- OXYGEN COMPRESSED LABEL
- GENERAL WARNINGS AND PRECAUTIONS
- OXYGEN REFRIGERATED LIQUID LABEL
- OXYGEN CERTIFICATE OF ANALYSIS
FULL PRESCRIBING INFORMATION
OXYGEN COMPRESSED LABEL
OXYGEN COMPRESSED USP UN 1072
PRODUCED BY AIR LIQUEFACTION
WARNING: HIGH PRESSURE OXIDIZING GAS VIGOROUSLY ACCELERATES COMBUSTION. KEEP OIL AND GREASE AWAY. OPEN VALVE SLOWLY. STORE AND USE WITH ADEQUATE VENTILATION. USE ONLY WITH EQUIPMENT CLEANED FOR OXYGEN SERVICE AND RATED FOR CYLINDER PRESSURE. USE ONLY WITH PRESSURE REDUCING EQUIPMENT AND APPARATUS DESIGNED FOR OXYGEN SERVICE. USE A BACK FLOW PREVENTATIVE IN THE PIPING. CLOSE VALVE AFTER EACH USE AND WHEN EMPTY. CYLINDER TEMPERATURES SHOULD NOT EXCEED 52 C (125 F) USE IN ACCORDANCE WITH MATERIAL SAFETY DATA SHEET (MSDS)
 .
.
GENERAL WARNINGS AND PRECAUTIONS
Warning: for emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications Rx Only. Uninterrupted use of high concentrations of oxygen over a long duration without monitoring oxygen content of arterial blood may be harmful. Uninterrupted use of high concentrations of oxygen for more than 5 hours may be harmful. Do not attempt to use on patients who have stopped breathing, unless used in conjunction with resuscitative equipment.
DO NOT REMOVE THIS PRODUCT LABEL
OXYGEN REFRIGERATED LIQUID LABEL
OXYGEN REFRIGERATED LIQUID U.S.P. UN 1073
ALWAYS KEEP CONTAINER IN UPRIGHT POSITION
WARNING: EXTREMELY COLD OXIDIZING LIQUID AND GAS UNDER PRESSURE. VIGOROUSLY ACCELERATES COMBUSTION. COMBUSTIBLES IN CONTACT WITH LIQUID OXYGEN MAY EXPLODE ON IGNITION OR IMPACT. CAN CAUSE SEVERE FROSTBITE. STORE AND USE WITH ADEQUATE VENTILATION. KEEP OIL, GREASE, AND COMBUSTIBLES AWAY. NO SMOKING IN CONTAINER AREA. DO NOT USE OR STORE NEAR HEAT OR OPEN FLAME. USE ONLY WITH EQUIPMENT CLEANED FOR OXYGEN SERVICE. DO NOT GET LIQUID IN EYES ON SKIN OR CLOTHING. FOR LIQUID WITHDRAWAL, WEAR FACE SHIELD AND GLOVES. DO NOT DROP USE HAND TRUCK FOR CONTAINER MOVEMENT. AVOID SPILLS. DO NOT WALK ON OR ROLL EQUIPMENT OVER SPILLS. CLOSE VALVE AFTER EACH USE AND WHEN EMPTY. USE IN ACCORDANCE WITH MATERIAL SAFETY DATA SHEET (MSDS).
FIRST AID: IN CASE OF FROSTBITE OBTAIN MEDICAL TREATMENT IMMEDIATELY.

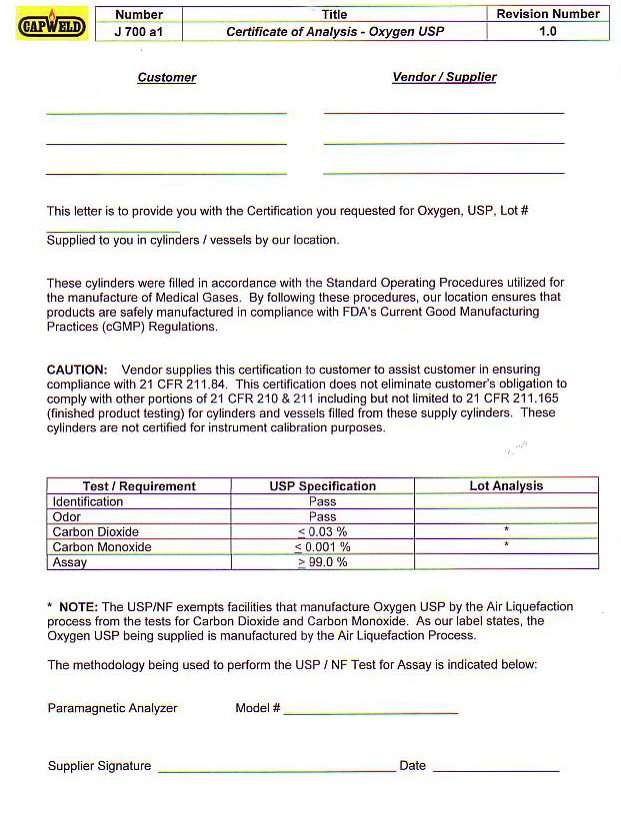
OXYGEN CERTIFICATE OF ANALYSIS
CUSTOMER ___________________ VENDOR / SUPPLIER ________________________ THIS LETTER IS TO PROVIDE YOU WITH THE
CERTIFICATION YOU REQUESTED FOR OXYGEN USP LOT NUMBER _______________________ SUPPLIED TO YOU IN
CYLINDERS / VESSELS BY OUR LOCATION
These cylinders were filled in accordance with the Standard Operating
Procedures utilized for the manufacture of Medical Gases. By
following these procedures, our location ensures that products are
safely manufactured in compliance with FDA’s Current Good Manufacturing
Practices
(cGMP) Regulations.
CAUTION: VENDOR SUPPLIES THIS CERTIFICATION TO CUSTOMER TO ASSIST
CUSTOMER IN ENSURING COMPLIANCE WITH 21 CFR 211.84. THIS
CERTIFICATION DOES NOT ELIMINATE CUSTOMERS OBLIGATION TO COMPLY WITH
OTHER PORTIONS OF 21 CFR 210 AND 211, INCLUDING BUT NOT
LIMITED TO 21 CFR 211.165 (FINISHED PRODUCT TESTING) FOR CYLINDERS AND
VESSELS FILLED FROM THESE SUPPLY CYLINDERS. THESE CYLINDERS ARE
NOT CERTIFIED FOR INSTRUMENT CALIBRATION.
TEST REQUIREMENT USP SPECIFICATION LOT ANALYSIS
IDENTIFICATION PASS
ODOR PASS
CARBON DIOXIDE LESS THAT 0.03%
CARBON MONOXIDE LESS THAT 0.001%
ASSAY GREATER THAN 99.0%
*
NOTE: The USP/NF exempts facilities that manufacture Oxygen USP by the
Air Liquefaction process from the tests for Carbon Dioxide and Carbon
Monoxide.
As our label states, the Oxygen USP being supplied is manufactured by the Air Liquefaction Process.
The methodology being used to perform the USP / NF Test for
Assay is indicated below: Paramagnetic Analyzer Model # _________________________
J 700 a1
REVISION 1.0
CERTIFICATE OF ANALYSIS - OXYGEN USP
OXYGENOXYGEN GAS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||