Pamine
PharmaDerm a division of Fougera Pharmaceuticals Inc.
Pamine 2.5 mg/Pamine Forte 5 mg
FULL PRESCRIBING INFORMATION: CONTENTS*
- PAMINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PAMINE INDICATIONS AND USAGE
- PAMINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PAMINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- PAMINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 100 tablets
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 60 tablets
FULL PRESCRIBING INFORMATION
PAMINE DESCRIPTION
Pamine® 2.5 mg/Pamine® Forte 5 mg Tablets contain methscopolamine bromide, an anticholinergic, which occurs as white crystals, or as a white odorless crystalline powder. Methscopolamine bromide melts at about 225°C with decomposition. The drug is freely soluble in water, slightly soluble in alcohol, and insoluble in acetone and in chloroform.
The chemical name for methscopolamine bromide is 3-Oxa-9-azoniatricyclo [3.3.1.02,4]nonane, 7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9, 9-dimethyl-, bromide, [7(S)-(1α, 2β, 4β, 5α, 7β)]- and the molecular weight is 398.30.
The structural formula is represented below:
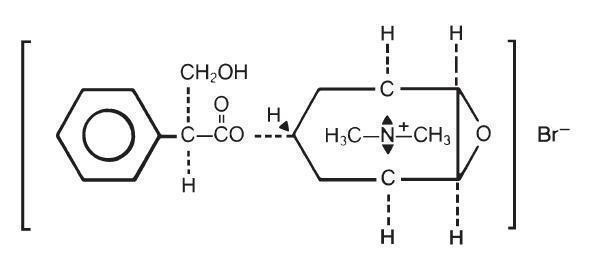
Pamine® 2.5 mg Tablets for oral administration contain 2.5 mg of methscopolamine bromide. Pamine® Forte 5 mg Tablets for oral administration contain 5 mg of methscopolamine bromide.
Inactive ingredients: microcrystalline cellulose, pregelatinized starch, magnesium stearate.
Contains no lactose.
CLINICAL PHARMACOLOGY
Methscopolamine bromide is an anticholinergic agent which possesses most of the pharmacologic actions of that drug class. These include reduction in volume and total acid content of gastric secretion, inhibition of gastrointestinal motility, inhibition of salivary excretion, dilation of the pupil and inhibition of accommodation with resulting blurring of vision. Large doses may result in tachycardia.
PHARMACOKINETICS
Methscopolamine bromide is a quaternary ammonium derivative of scopolamine. As a class, these agents are poorly and unreliably absorbed.1,2 Total absorption of quaternary ammonium derivatives of the alkaloids is 10-25%. Rate of absorption is not available. Quaternary ammonium salts have limited absorption from intact skin, and conjunctival penetration is poor.1 Little is known of the fate and excretion of most of these agents.1 Following oral administration, drug effects appear in about one hour and persist for 4 to 6 hours.2 Methscopolamine bromide has limited ability to cross the blood-brain barrier.3,4,5 The drug is excreted primarily in the urine and bile, or as unabsorbed drug in feces.2 There is no data on the presence of methscopolamine in breast milk; traces of atropine have been found after administration of atropine.1
PAMINE INDICATIONS AND USAGE
Adjunctive therapy for the treatment of peptic ulcer.
METHSCOPOLAMINE BROMIDE HAS NOT BEEN SHOWN TO BE EFFECTIVE IN CONTRIBUTING TO THE HEALING OF PEPTIC ULCER, DECREASING THE RATE OF RECURRENCE OR PREVENTING COMPLICATIONS.
PAMINE CONTRAINDICATIONS
Glaucoma; obstructive uropathy (e.g., bladder neck obstruction due to prostatic hypertrophy); obstructive disease of the gastrointestinal tract (e.g., pyloroduodenal stenosis); paralytic ileus; intestinal atony of the elderly or debilitated patient; unstable cardiovascular status in acute hemorrhage; severe ulcerative colitis; toxic megacolon complicating ulcerative colitis; myasthenia gravis.
Pamine® 2.5 mg/Pamine® Forte 5 mg is contraindicated in patients who are hypersensitive to methscopolamine bromide or related drugs.
WARNINGS
In the presence of high environmental temperature, heat prostration (fever and heat stroke due to decreased sweating) can occur with drug use.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance treatment with this drug would be inappropriate and possibly harmful.
Methscopolamine bromide may produce drowsiness or blurred vision. The patient should be cautioned regarding activities requiring mental alertness such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
With overdosage, a curare-like action may occur, i.e., neuromuscular blockade leading to muscular weakness and possible paralysis.
PRECAUTIONS
1. General precautions
Use Pamine® 2.5 mg/Pamine® Forte 5 mg Tablets with caution in the elderly and in all patients with: autonomic neuropathy; hepatic or renal disease; or ulcerative colitis –large doses may suppress intestinal motility to the point of producing a paralytic ileus and for this reason precipitate or aggravate "toxic megacolon," a serious complication of the disease.
The drug also should be used with caution in patients having hyperthyroidism, coronary heart disease, congestive heart failure, tachyrhythmia, tachycardia, hypertension, or prostatic hypertrophy.
2. Information for patient
See statement under WARNINGS.
3. Laboratory tests
Progress of the peptic ulcer under treatment should be followed by upper gastrointestinal contrast radiology or endoscopy to insure healing. Stool tests for occult blood and blood hemoglobin or hematocrit values should be followed to rule out bleeding from the ulcer.
4. Drug interactions
Additive anticholinergic effects may result from concomitant use with antipsychotics, tricyclic antidepressants, and other drugs with anticholinergic effects. Concomitant administration with antacids may interfere with the absorption of methscopolamine bromide.
5. Carcinogenesis, mutagenesis, impairment of fertility
No long-term studies in animals have been performed to evaluate carcinogenic potential.
6. Pregnancy
Teratogenic effects
Pregnancy Category C
Animal reproduction studies have not been conducted with methscopolamine bromide. It also is not known whether methscopolamine bromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methscopolamine bromide should be given to a pregnant woman only if clearly needed.
7. Nursing mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when methscopolamine bromide is administered to a nursing woman.
Anticholinergic drugs may suppress lactation.
8. Pediatric use
Safety and efficacy in children have not been established.
PAMINE ADVERSE REACTIONS
The following adverse reactions have been observed, but there is not enough data to support an estimate of frequency.
Cardiovascular: Tachycardia, palpitation.
Allergic: Severe allergic reaction or drug idiosyncrasies including anaphylaxis.
CNS: Headaches, nervousness, mental confusion, drowsiness, dizziness.
Special Senses: Blurred vision, dilation of the pupil, cycloplegia, increased ocular tension, loss of taste.
Renal: Urinary hesitancy and retention.
Gastrointestinal: Nausea, vomiting, constipation, bloated feeling.
Dermatologic: Decreased sweating, urticaria and other dermal manifestations.
Miscellaneous: Xerostomia, weakness, insomnia, impotence, suppression of lactation.
DRUG ABUSE AND DEPENDENCE
Not applicable.
OVERDOSAGE
The symptoms of overdosage with Pamine® 2.5 mg/Pamine® Forte 5 mg Tablets progress from intensification of the usual side effects to CNS disturbances (from restlessness and excitement to psychotic behavior), circulatory changes (flushing, fall in blood pressure, circulatory failure), respiratory failure, paralysis, and coma.
Measures to be taken are (1) induction of emesis and (2) injection of physostigmine 0.5 to 2 mg intravenously, and repeated as necessary up to a total of 5 mg. Fever may be treated symptomatically (alcohol sponging, ice packs). Excitement of a degree which demands attention may be managed with sodium thiopental 2% solution given slowly intravenously or chloral hydrate (100-200 mL of a 2% solution) by rectal infusion. In the event of progression of the curare-like effect to paralysis of the respiratory muscles, artificial respiration should be instituted and maintained until effective respiratory action returns.
The oral LD50 in rats is 1,352 to 2,617 mg/kg.
No data is available on the dialyzability of methscopolamine bromide.
PAMINE DOSAGE AND ADMINISTRATION
The average dosage of Pamine® Tablets is 2.5 mg one-half hour before meals and 2.5 to 5 mg at bedtime. A starting dose of 12.5 mg daily will be clinically effective in most patients without the production of appreciable side effects.
If the patient is experiencing symptoms such as severe abdominal pain or cramping which demand prompt relief, the drug may be started on a daily dosage of 20 mg, administered in doses of 5 mg one-half hour before meals and at bedtime. If very unpleasant side effects develop promptly, the daily dosage should be reduced. If neither symptomatic relief nor side effects appear, the daily dosage may be increased. Some patients have tolerated 30 mg daily with no unpleasant reactions.
Patients whose dosage has been reduced to eliminate or modify side effects often continue to show adequate response both subjectively in relief of symptoms and objectively as measured by antisecretory effects.
The ultimate aim of therapy is to arrive at a dosage which provides maximal clinical effectiveness with a minimum of unpleasant side effects. Many patients report no side effects on a dosage which gives complete relief of symptoms. On the other hand, some patients have reported severe side effects without appreciable symptomatic relief. Such patients must be considered unsuited for this therapy. Usually they have been or will prove to be similarly intolerant to other anticholinergic drugs. If methscopolamine bromide is to be used in a patient who gives a history of such intolerance, it should be started at a low dosage.
HOW SUPPLIED
Pamine® 2.5 mg Tablets are available as white, round tablets, debossed with "PAMINE" on one side, in the following package size:
Bottles of 100 (NDC 10337-061-01)
Pamine® Forte 5 mg Tablets are available as white, oval tablets, debossed with "PAMINE 5" on one side, in the following package size:
Dose Pack (5 blisters of 12 tablets)
Box of 60 (NDC 10337-062-06)
Store at controlled room temperature 15°-30°C (59°-86°F). KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
REFERENCES
- Gilman A, Gilman AB, Goodman LA, eds.
The Pharmacological Basis of Therapeutics.
6th ed. New York: MacMillan Publishing Company.1980. - American Hospital Formulary Service. American Society of Hospital Pharmacists. Bethesda, Maryland.
- Domino EF, Corasen G. Central and Peripheral Effects of Muscarinic Cholinergic Blocking Agents in Man. Anesthesiology 1967;28:568-574.
- Mogensen L, Orinius E. Arrhythmic Complications after Parasympathetic Treatment of Bradyarrhythmias in a Coronary Care Unit. Acta Med Scand 1971;190:495-498.
- Neeld JB Jr., et al. Cardiac Rate and Rhythm Changes with Atropine and Methscopolamine. Clin Pharmacol Ther 1975;17(3):290-295.
Rx Only
Manufactured for:
PharmaDerm®
A division of Fougera Pharmaceuticals Inc.
Melville, NY 11747 USA
www.pharmaderm.com
Mfd. by:
Mikart, Inc., Atlanta, GA 30318 USA
IL175B R2/12
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 100 tablets
NDC 10337-061-01
100 Tablets
Rx Only
Pamine®
(methscopolamine bromide)
2.5 mg
PharmaDerm®
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 60 tablets
NDC 10337-062-06
Dose Pack
Dispense As One Unit
Rx Only
Pamine®
Forte 5mg
(methscopolamine bromide)
60 Tablets (5 blisters of 12 tablets)
PharmaDerm®
Paminemethscopolamine bromide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pamine Fortemethscopolamine bromide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||