PANCRELIPASE
FULL PRESCRIBING INFORMATION: CONTENTS*
- PANCRELIPASE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PANCRELIPASE INDICATIONS AND USAGE
- PANCRELIPASE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PANCRELIPASE ADVERSE REACTIONS
- PANCRELIPASE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
PANCRELIPASE DESCRIPTION
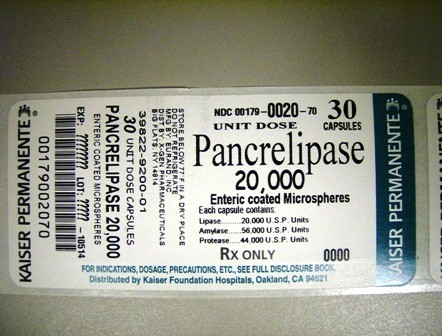
Pancrelipase 20,000 capsules are orally administered and contain delayed-release microspheres of porcine pancreatic enzyme concentrate, predominantly pancreatic lipase, amylase and protease.
Each 20,000 capsule contains:
Lipase 20,000 USP Units
Amylase 56,000 USP Units
Protease 44,000 USP Units
Inactive Ingredients:
Diethyl Phthalate NF, Hydroxypropyl Methylcellulose USP, Hypromellose Phthalate NF, Polyethylene Glycol NF, and Povidone USP.
CLINICAL PHARMACOLOGY
Pancrelipase capsules resist gastric inactivation and deliver predicable, high levels of biologically active enzymes into the duodenum. The enzymes catalyze the hydrolysis of fats into glycerol and fatty acids, protein into proteoses and derived substances, and starch into dextrins and sugars. Pancrelipase capsules are effective in controlling steatorrhea and its consequences at low daily dosage levels.
PANCRELIPASE INDICATIONS AND USAGE
Pancrelipase capsules are indicated for patients with exocrine pancreatic enzyme deficiency as in but not limited to: cystic fibrosis, chronic pancreatitis, post-pancreatectomy, post-gastrointestinal bypass surgery (e.g. Billroth II gastroenterostomy), ductal obstruction from neoplasm (e.g. of the pancreas or common bile duct).
PANCRELIPASE CONTRAINDICATIONS
Pancrelipase capsules are contraindicated in patients known to be hypersensitive to pork protein.
Pancrelipase capsules are contraindicated in patients with acute pancreatitis or with acute exacerbations of chronic pancreatic diseases.
WARNINGS
Should hypersensitivity occur, discontinue medication and treat symptomatically.
PRECAUTIONS
TO PROTECT ENTERIC COATING, MICROSPHERES SHOULD NOT BE CRUSHED OR CHEWED.
Where swallowing of capsules is difficult, they may be opened and the microspheres shaken into a small quantity of a soft food (e.g. applesauce, gelatin, etc.), which does not require chewing, and swallowed immediately. Contact of the microspheres with foods having a pH greater than 5.5 can dissolve the protective enteric shell.
Pregnancy Category C.
Diethylphthalate, an enteric coating component of
pancrelipase capsules has been shown with high intraperitoneal dosing
to be teratogenic in rats. However, when this coating was administered
orally to rats up to 100 times the human dose, no teratogenic or
embryocidal effects were observed. There were no adequate and
well-controlled studies in pregnant women. Pancrelipase capsules should
be used in pregnancy only if the potential benefit justifies the
potential risk to the fetus.
Cases of fibrotic stricture in the ascending colon have been reported in cystic fibrosis patients with the use of enzyme supplements in high doses (approximately 6,500 - 50,000 USP lipase units/kg/meal). If symptoms suggestive to gastrointestinal obstruction occur, the possibility of bowl strictures should be considered.
PANCRELIPASE ADVERSE REACTIONS
PANCRELIPASE DOSAGE AND ADMINISTRATION
Dosage should be adjusted according to the severity of the exocrine pancreatic enzyme deficiency. The number of capsules or capsule strength given with meals and/or snacks should be estimated by assessing which dose minimizes steatorhea and maintains good nutritional status. Dose increases if required, should be made slowly, with careful monitoring of response and symptomatology. It is important to ensure adequate hydration of patients at all times while dosing Pancrelipase capsules. In some patients with pancreatic enzyme deficiency, satisfactory responses have been achieved with dosages (expressed in U.S.P. units of lipase) similar to the ones stated below. However, dosages should be adjusted according to the response of the patient.
Children 7 to 12 years: 4,000 to 12,000 units (more if necessary) with each meal and with snacks.
Children 1 to 6 years: 4,000 to 8,000 units with each meal and 4,000 units with snacks.
Children under 1 year: Dosage for children under 6 months of age has not been established. Children 6 months to 1 year have responded to 2,000 units of lipase per meal.
The assessment of the end points in children is aided by charting growth curves.
Adults: 4,000 to 20,000 units (more if necessary) with each meal and with snacks.
HOW SUPPLIED
PANCRELIPASE 20,000 Capsules- flesh, natural; printed EUR 20,000 30 capsules in 1 Box, Unit-Dose.
Store below 25°C (77°F) in a dry place.
Do not refrigerate.
No studies have been performed to determine equivalence of this product with any other products with the same or similar co-enzyme content or ratio and no such claims are intended.
Rx only.
Manufactured by:
EURAND Inc.
Vandalia, OH 45377
Distributed by:
X-GEN Pharmaceuticals, Inc.
Big Flats, NY 14814
Repackaged by:
KAISER FOUNDATION HOSPITALS
Livermore, CA 94551
PANCRELIPASEPANCRELIPASE CAPSULE, DELAYED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||