Paremyd
PAREMYD
FULL PRESCRIBING INFORMATION: CONTENTS*
- PAREMYD DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- PAREMYD CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PAREMYD ADVERSE REACTIONS
- OVERDOSAGE
- PAREMYD DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
1%/0.25%
Sterile
Rx only
PAREMYD DESCRIPTION
PAREMYD® sterile ophthalmic solution is a topical mydriatic combination product for ophthalmic use.
STRUCTURAL FORMULAE
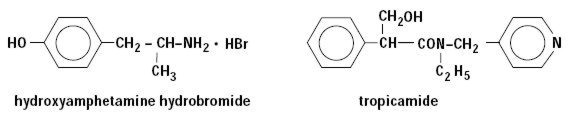
CHEMICAL NAME
Hydroxyamphetamine hydrobromide: Phenol, 4-(2-aminopropyl)-, hydrobromide
Tropicamide: Benzeneacetamide, N-ethyl-α-(hydroxymethyl)-N-(4-pyridinylmethyl)-
CONTAINS
Actives: Hydroxyamphetamine hydrobromide, USP.......................1.0%
Tropicamide, USP.................................................................0.25%
Preservative: Benzalkonium Chloride 0.005%
Inactives: Edetate Disodium 0.015%, Sodium Chloride; Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (4.2 to 5.8 during its shelf life), and Purified Water. The osmolality of PAREMYD® is approximately 307 mOsm/l.
CLINICAL PHARMACOLOGY
PAREMYD® Solution combines the effects of the adrenergic agent, hydroxyamphetamine hydrobromide, and the anticholinergic agent, tropicamide.
Hydroxyamphetamine hydrobromide is an indirectly-acting sympathomimetic agent which, when applied topically to the eye, causes the release of endogenous norepinephrine from intact adrenergic nerve terminals resulting in mydriasis. Since hydroxyamphetamine hydrobromide has little or no direct activity on the receptor site, dilation does not usually occur if there is damage to the presynaptic nerve terminal, e.g., Horner's Syndrome. However, it is not known whether damage to the presynaptic nerve terminal will influence the extent of mydriasis produced by PAREMYD®. Hydroxyamphetamine hydrobromide has minimal cycloplegic action.
Tropicamide is a parasympatholytic agent which, when applied topically to the eye, blocks the responses of the sphincter muscle of the iris and the ciliary muscle to cholinergic stimulation, producing dilation of the pupil and paralysis of the ciliary muscle. Tropicamide produces short-duration mydriasis. Although cycloplegia occurs with higher doses of tropicamide, there is evidence with 0.25% tropicamide that full cycloplegia does not occur.
Since both these agents act on different effector sites, their simultaneous use produces an additive mydriatic effect. PAREMYD® provides diminished pupil responsiveness to light, facilitating ophthalmoscopy. The onset of action with PAREMYD® occurs within 15 minutes, followed by maximum effect within 60 minutes after instillation of one drop. Clinically significant dilation, inhibition of pupillary light response, and partial cycloplegia last 3 hours, with recovery beginning at approximately 90 minutes and with complete recovery occurring in most patients in 6 to 8 hours. However, in some cases complete recovery may take up to 24 hours. Effectiveness may differ slightly in patients with light and dark irides, with those patients with light irides experiencing a slightly greater mydriasis.
INDICATIONS
PAREMYD® Solution is indicated for mydriasis in routine diagnostic procedures and in conditions where short-term pupil dilation is desired. PAREMYD® provides clinically significant mydriasis with partial cycloplegia.
PAREMYD CONTRAINDICATIONS
PAREMYD® Solution should not be used in patients with angle-closure glaucoma or in those with narrow angles in whom dilation of the pupil may precipitate an attack of angle-closure glaucoma. This product is also contraindicated in patients who are hypersensitive to any of its components.
WARNINGS
For topical ophthalmic use only; not for injection.
There is evidence that mydriatics may produce a transient elevation of intraocular pressure in patients with open-angle glaucoma.
This preparation rarely may cause CNS disturbances which may be particularly dangerous in infants, children or the aged. Psychotic reactions, behavioral disturbances and vasomotor or cardio-respiratory collapse have been reported with the use of anticholinergic drugs.
PRECAUTIONS
General
Patients with hypertension, hyperthyroidism, diabetes or cardiac disease (i.e., arrhythmias or chronic ischemic heart disease) should be monitored after instillation. The elderly and others in whom glaucoma or increased intraocular pressure may be encountered following administration of PAREMYD® Solution should also be monitored closely. To avoid inducing angle-closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made.
Information for Patients
Patients should be advised not to touch the dropper tip to any surface since this may contaminate the solution. Patients should be advised to use caution when driving or engaging in other hazardous activities while pupils are dilated. Patients may experience photophobia and/or blurred vision and should protect their eyes in bright illumination when pupils are dilated. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been performed to evaluate the carcinogenic, mutagenic or impairment of fertility potential of PAREMYD®.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with PAREMYD®. It is also not known whether PAREMYD® can cause fetal harm when administered to a pregnant woman or can affect reproduction capability. PAREMYD® should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PAREMYD® is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. PAREMYD® may rarely cause CNS disturbances which may be dangerous in infants and children. Psychotic reactions, behavioral disturbances and vasomotor or cardio-respiratory collapse in children have been reported with the use of anticholinergic drugs. (See WARNINGS ). Keep this and all medications out of the reach of children.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
PAREMYD ADVERSE REACTIONS
Increased intraocular pressure has been reported following use of mydriatics. Transient stinging, dryness of the mouth, blurred vision, photophobia with or without corneal staining, tachycardia, headache, allergic reactions, nausea, vomiting, pallor and muscle rigidity have been reported with the use of tropicamide and/or hydroxyamphetamine hydrobromide, and thus may occur with PAREMYD® Solution. Central nervous system disturbances have also been reported. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse have been reported with the use of anticholinergic drugs.
Rare but serious cardiovascular events, including death due to myocardial infarction, ventricular fibrillation and significant hypotensive episodes have occurred shortly following PAREMYD® instillation.
OVERDOSAGE
Ocular overdosage will cause dilation of the pupils. Systemic overdosage or ingestion of large doses may result in hypertension, cardiac arrhythmias, sub-sternal discomfort, headache, sweating, nausea, vomiting and gastrointestinal irritation. Patients with systemic overdosage should be carefully monitored and treated symptomatically.
PAREMYD DOSAGE AND ADMINISTRATION
One to two drops in the conjunctival sac. The onset of action with PAREMYD® Solution occurs within 15 minutes followed by maximum effect within 60 minutes. Clinically significant dilation, inhibition of pupillary light response, and partial cycloplegia last 3 hours.
Mydriasis will reverse spontaneously with time, typically in 6 to 8 hours. However, in some cases, complete recovery may take up to 24 hours.
HOW SUPPLIED
PAREMYD® (hydroxyamphetamine hydrobromide/tropicamide ophthalmic solution) 1%/0.25% as a 15 mL solution in a 15 mL opaque white, low density polyethylene bottle with a natural low density polyethylene dropper tip and a red polypropylene cap.
15 mL - NDC 17478-704-12
STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Note: Protect from light.
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045
PM00N
Rev. 07/2012
Container Label Principal Display Panel
NDC 17478-704-12
PAREMYD®
(hydroxyamphetamine
hydrobromide/tropicamide
ophthalmic solution) 1%/0.25%
15 mL Sterile Rx only
Carton Principal Display Panel
NDC 17478-704-12
[Akorn logo]
PAREMYD®
(hydroxyamphetamine
hydrobromide/tropicamide
ophthalmic solution)
1%/0.25%
15 mL
Sterile Rx only
ParemydHydroxyamphetamine hydrobromide, tropicamide SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||