Paromomycin Sulfate
Caraco Pharmaceutical Laboratories, Ltd.
FULL PRESCRIBING INFORMATION: CONTENTS*
- PAROMOMYCIN SULFATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PAROMOMYCIN SULFATE INDICATIONS AND USAGE
- PAROMOMYCIN SULFATE CONTRAINDICATIONS
- PRECAUTIONS
- PAROMOMYCIN SULFATE ADVERSE REACTIONS
- PAROMOMYCIN SULFATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL – 250 mg (100 count)
FULL PRESCRIBING INFORMATION
PAROMOMYCIN SULFATE DESCRIPTION
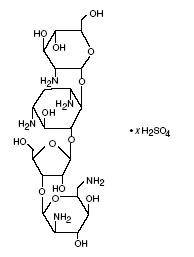
Paromomycin sulfate is a broad spectrum antibiotic produced by Streptomyces riomosus var. paromomycinus. It is a white, amorphous, stable, water-soluble product. Paromomycin sulfate is designated chemically as O-2,6-Diamino-2,6-dideoxy-β-L-idopyranosyl-(1→3)-O-β-D-ribofuranosyl(1→5)-O-[2-amino-2-deoxy-α-D-glucopyranosyl-(1→4)]-2-deoxystreptamine sulfate (salt). The molecular formula is C23H45N5O14 • xH2SO4, with a molecular weight of 615.64 (base). Its structural formula is:
Each capsule, for oral administration, contains paromomycin sulfate equivalent to 250 mg paromomycin. Each capsule also contains the following inactive ingredients: FD&C Green #3; FD&C Yellow #5 (tartrazine); gelatin, NF; and titanium dioxide, USP.
CLINICAL PHARMACOLOGY
The in vitro and in vivo antibacterial action of paromomycin closely parallels that of neomycin. It is poorly absorbed after oral administration, with almost 100% of the drug recoverable in the stool.
PAROMOMYCIN SULFATE INDICATIONS AND USAGE
Paromomycin sulfate is indicated for intestinal amebiasis—acute and chronic (NOTE—It is not effective in extraintestinal amebiasis); management of hepatic coma—as adjunctive therapy.
PAROMOMYCIN SULFATE CONTRAINDICATIONS
Paromomycin sulfate is contraindicated in individuals with a history of previous hypersensitivity reactions to it. It is also contraindicated in intestinal obstruction.
PRECAUTIONS
The use of this antibiotic, as with other antibiotics, may result in an overgrowth of nonsusceptible organisms, including fungi. Constant observation of the patient is essential. If new infections caused by nonsusceptible organisms appear during therapy, appropriate measures should be taken.
The drug should be used with caution in individuals with ulcerative lesions of the bowel to avoid renal toxicity through inadvertent absorption.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
PAROMOMYCIN SULFATE ADVERSE REACTIONS
Nausea, abdominal cramps, and diarrhea have been reported in patients on doses over 3 g daily.
PAROMOMYCIN SULFATE DOSAGE AND ADMINISTRATION
Intestinal amebiasis: Adults and Children: Usual dose—25 to 35 mg/kg body weight daily, administered in three doses with meals, for five to ten days.
Management of hepatic coma: Adults: Usual dose—4 g daily in divided doses, given at regular intervals for five to six days.
HOW SUPPLIED
Paromomycin Sulfate Capsules, each contain paromomycin sulfate equivalent to 250 mg paromomycin. The capsule is green/yellow, imprinted “175” in black ink on the cap and body.
NDC 57664-175-08: Bottles of 100
Store at controlled room temperature 15°-30°C (59°-86°F).
Protect from moisture.
Caution—Federal law prohibits dispensing without prescription.
Manufactured by: C.S. No.: 5106T02
Caraco Pharmaceutical Laboratories, Ltd. Iss.: 11/08
1150 Elijah McCoy Drive
Detroit, MI 48202
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL – 250 mg (100 count)
NDC 57664-175-08
Paromomycin Sulfate Capsules, USP
250 mg*
Rx Only
100 Capsules
Caraco Pharmaceutical Laboratories, Ltd.

Paromomycin SulfateParomomycin Sulfate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||