Paroxetine
State of Florida DOH Central Pharmacy
FULL PRESCRIBING INFORMATION: CONTENTS*
- Suicidality in Children and Adolescents
- PAROXETINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PAROXETINE INDICATIONS AND USAGE
- PAROXETINE CONTRAINDICATIONS
- WARNINGS
- Clinical Worsening and Suicide Risk
- Screening Patients for Bipolar Disorder
- Potential for Interaction With Monoamine Oxidase Inhibitors
- Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
- Potential Interaction With Thioridazine
- Usage in Pregnancy
- Teratogenic Effects
- Animal Findings
- Nonteratogenic Effects
- PRECAUTIONS
- General
- Activation of Mania/Hypomania
- Seizures
- Discontinuation of Treatment With Paroxetine Tablets
- Akathisia
- Hyponatremia
- Abnormal Bleeding
- Use in Patients With Concomitant Illness
- Information for Patients
- Clinical Worsening and Suicide Risk
- Drugs That Interfere With Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
- Interference With Cognitive and Motor Performance
- Completing Course of Therapy
- Concomitant Medication
- Alcohol
- Pregnancy
- Nursing
- Laboratory Tests
- Drug Interactions
- Tryptophan
- PAROXETINE ADVERSE REACTIONS
- Associated With Discontinuation of Treatment
- Commonly Observed Adverse Events
- Major Depressive Disorder
- Obsessive Compulsive Disorder
- Panic Disorder
- Generalized Anxiety Disorder
- Incidence in Controlled Clinical Trials
- Major Depressive Disorder
- Obsessive Compulsive Disorder and Panic Disorder
- Generalized Anxiety Disorder
- Dose Dependency of Adverse Events
- Adaptation to Certain Adverse Events
- Male and Female Sexual Dysfunction With SSRIs
- Weight and Vital Sign Changes
- ECG Changes
- Liver Function Tests
- Hallucinations
- Other Events Observed During the Premarketing Evaluation of Paroxetine
- Body as a Whole
- Cardiovascular System
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- PAROXETINE DOSAGE AND ADMINISTRATION
- Major Depressive Disorder
- Usual Initial Dosage
- Maintenance Therapy
- Obsessive Compulsive Disorder
- Usual Initial Dosage
- Maintenance Therapy
- Panic Disorder
- Usual Initial Dosage
- Maintenance Therapy
- Generalized Anxiety Disorder
- Usual Initial Dosage
- Maintenance Therapy
- Special Populations
- Treatment of Pregnant Women During the Third Trimester
- Dosage for Elderly or Debilitated Patients, and Patients With Severe Renal or Hepatic Impairment
- Switching Patients to or From a Monoamine Oxidase Inhibitor
- Discontinuation of Treatment With Paroxetine Tablets
- HOW SUPPLIED
- MEDICATION GUIDE
- 20mg Label
- 30mg Label
- 40mg Label
FULL PRESCRIBING INFORMATION
Suicidality in Children and Adolescents
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of paroxetine tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Paroxetine is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.)
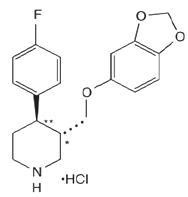
PAROXETINE DESCRIPTION
transRS192032
CLINICAL PHARMACOLOGY
Pharmacodynamics
In vitroIn vitro1221211
Pharmacokinetics
Absorption and Distribution
maxmaxmin½maxmin0-24
max
in vitro
Metabolism and Excretion
min
PRECAUTIONS
Other Clinical Pharmacology Information
Specific Populations
Renal and Liver Disease:max
DOSAGE AND ADMINISTRATION
Elderly Patients:minmin DOSAGE AND ADMINISTRATION
Drug-Drug Interactions
In vitro PRECAUTIONS—Drug Interactions
Clinical Trials
Major Depressive Disorder
Obsessive Compulsive Disorder
| Outcome Classification |
Placebo (n = 74) |
Paroxetine 20 mg (n = 75) |
Paroxetine 40 mg (n = 66) |
Paroxetine 60 mg (n = 66) |
|---|---|---|---|---|
| Worse |
14% |
7% |
7% |
3% |
| No Change |
44% |
35% |
22% |
19% |
| Minimally Improved |
24% |
33% |
29% |
34% |
| Much Improved |
11% |
18% |
22% |
24% |
| Very Much Improved |
7% |
7% |
20% |
20% |
Panic Disorder
Generalized Anxiety Disorder
PAROXETINE INDICATIONS AND USAGE
Major Depressive Disorder
CLINICAL PHARMACOLOGY—Clinical Trials
CLINICAL PHARMACOLOGY—Clinical Trials
Obsessive Compulsive Disorder
CLINICAL PHARMACOLOGY—Clinical Trials
CLINICAL PHARMACOLOGY—Clinical Trials DOSAGE AND ADMINISTRATION
Panic Disorder
CLINICAL PHARMACOLOGY—Clinical Trials
CLINICAL PHARMACOLOGY—Clinical Trials
Generalized Anxiety Disorder
CLINICAL PHARMACOLOGY—Clinical Trials
CLINICAL PHARMACOLOGY—Clinical Trials DOSAGE AND ADMINISTRATION
PAROXETINE CONTRAINDICATIONS
WARNINGS PRECAUTIONS
PRECAUTIONS
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated |
|---|---|
| Increases Compared to Placebo |
|
| <18 |
14 additional cases |
| 18-24 |
5 additional cases |
| Decreases Compared to Placebo |
|
| 25-64 |
1 fewer case |
| ≥65 |
6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms (see PRECAUTIONS and DOSAGE AND ADMINISTRATION — Discontinuation of Treatment with Paroxetine Tablets ), for a description of the risks of discontinuation of paroxetine.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for paroxetine should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
Potential for Interaction With Monoamine Oxidase Inhibitors
In patients receiving another serotonin reuptake inhibitor drug in combination with a monoamine oxidase inhibitor (MAOI), there have been reports of serious, sometimes fatal, reactions including hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have recently discontinued that drug and have been started on an MAOI. Some cases presented with features resembling neuroleptic malignant syndrome. While there are no human data showing such an interaction with paroxetine, limited animal data on the effects of combined use of paroxetine and MAOIs suggest that these drugs may act synergistically to elevate blood pressure and evoke behavioral excitation. Therefore, it is recommended that paroxetine not be used in combination with an MAOI (including linezolid, an antibiotic which is a reversible non-selective MAOI), or within 14 days of discontinuing treatment with an MAOI (see CONTRAINDICATIONS ). At least 2 weeks should be allowed after stopping paroxetine before starting an MAOI.
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported with SNRIs and SSRIs alone, including treatment with paroxetine, but particularly with concomitant use of serotonergic drugs (including triptans) with drugs which impair metabolism of serotonin (including MAOIs), or with antipsychotics or other dopamine antagonists. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
The concomitant use of paroxetine with MAOIs intended to treat depression is contraindicated.
If concomitant treatment of paroxetine with a 5-hydroxytryptamine receptor agonist (triptan) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases.
The concomitant use of paroxetine with serotonin precursors (such as tryptophan) is not recommended.
Treatment with paroxetine and any concomitant serotonergic or antidopaminergic agents, including antipsychotics, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Potential Interaction With Thioridazine
Thioridazine administration alone produces prolongation of the QTc interval, which is associated with serious ventricular arrhythmias, such as torsade de pointes–type arrhythmias, and sudden death. This effect appears to be dose related.
An in vivo study suggests that drugs which inhibit CYP2D6, such as paroxetine, will elevate plasma levels of thioridazine. Therefore, it is recommended that paroxetine not be used in combination with thioridazine (see CONTRAINDICATIONS and PRECAUTIONS).
Usage in Pregnancy
Teratogenic Effects
PRECAUTIONS—Discontinuation of Treatment With Paroxetine Tablets
Animal Findings
22
Nonteratogenic Effects
WARNINGS—Potential for Interaction With Monoamine Oxidase Inhibitors
Infants exposed to SSRIs in late pregnancy may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1 to 2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality. In a retrospective case-control study of 377 women whose infants were born with PPHN and 836 women whose infants were born healthy, the risk for developing PPHN was approximately six-fold higher for infants exposed to SSRIs after the 20th week of gestation compared to infants who had not been exposed to antidepressants during pregnancy. There is currently no corroborative evidence regarding the risk for PPHN following exposure to SSRIs in pregnancy; this is the first study that has investigated the potential risk. The study did not include enough cases with exposure to individual SSRIs to determine if all SSRIs posed similar levels of PPHN risk.
There have also been postmarketing reports of premature births in pregnant women exposed to paroxetine or other SSRIs.
DOSAGE AND ADMINISTRATION
PRECAUTIONS
General
Activation of Mania/Hypomania
Seizures
Discontinuation of Treatment With Paroxetine Tablets
DOSAGE AND ADMINISTRATION
PRECAUTIONS—Pediatric Use
Akathisia
Hyponatremia
Geriatric Use
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Abnormal Bleeding
Use in Patients With Concomitant Illness
DOSAGE AND ADMINISTRATION
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with paroxetineand should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions” is available for paroxetine. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking paroxetine.
Clinical Worsening and Suicide Risk
Drugs That Interfere With Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Interference With Cognitive and Motor Performance
Completing Course of Therapy
Concomitant Medication
Alcohol
Pregnancy
WARNINGS—Usage in Pregnancy: Teratogenic and Nonteratogenic Effects
Nursing
PRECAUTIONS—Nursing Mothers
Laboratory Tests
There are no specific laboratory tests recommended.
Drug Interactions
Tryptophan
WARNINGS–Serotonin Syndrome
Monoamine Oxidase Inhibitors
CONTRAINDICATIONS WARNINGS
Pimozide
maxmax CONTRAINDICATIONS
Serotonergic Drugs
WARNINGS—Serotonin Syndrome CONTRAINDICATIONS PRECAUTIONS—Drug Interactions, Tryptophan
Thioridazine
CONTRAINDICATIONS WARNINGS
Warfarin
Drugs That Interfere With Hemostasis
Triptans
WARNINGS–Serotonin Syndrome
Drugs Affecting Hepatic Metabolism
Cimetidine
450
Phenobarbital
450½
Phenytoin
½ ADVERSE REACTIONS—Postmarketing Reports
Drugs Metabolized by CYP2D6
450max½max
CONTRAINDICATIONS WARNINGS
450 PRECAUTIONS—Tricyclic Antidepressants
Drugs Metabolized by Cytochrome CYP3A4
in vivoin vitroin vitroiin vivo
Tricyclic Antidepressants (TCAs)
PRECAUTIONS—Drugs Metabolized by Cytochrome CYP2D6
Drugs Highly Bound to Plasma Protein
Drugs That Interfere With Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Alcohol
Lithium
Digoxin
Diazepam
Procyclidine
0-24maxmin
Beta-Blockers
ADVERSE REACTIONS—Postmarketing Reports
Theophylline
Fosamprenavir/Ritonavir
Electroconvulsive Therapy (ECT)
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
2
Mutagenesis
in vitroin vivoin vivoin vitro
Impairment of Fertility
22
Pregnancy
See WARNINGS—Usage in Pregnancy: Teratogenic and Nonteratogenic Effects .
Labor and Delivery
The effect of paroxetine on labor and delivery in humans is unknown.
Nursing Mothers
Pediatric Use
BOX WARNING and WARNINGS—Clinical Worsening and Suicide Risk
Discontinuation of Treatment With Paroxetine Tablets
Geriatric Use
PRECAUTIONS, Hyponatremia)
CLINICAL PHARMACOLOGY DOSAGE AND ADMINISTRATION
PAROXETINE ADVERSE REACTIONS
Associated With Discontinuation of Treatment
| |
Major Depressive Disorder |
OCD | Panic Disorder | Generalized Anxiety Disorder |
||||
|---|---|---|---|---|---|---|---|---|
| Paroxetine | Placebo | Paroxetine | Placebo | Paroxetine | Placebo | Paroxetine | Placebo | |
| Where numbers are not provided the incidence of the adverse events in patients treated with paroxetine was not >1% or was not greater than or equal to 2 times the incidence of placebo. 1. Incidence corrected for gender. |
||||||||
|
CNS
|
|
|
|
|
|
|
|
|
| Somnolence |
2.3% |
0.7% |
— |
|
1.9% |
0.3% |
2% |
0.2% |
| Insomnia |
— |
— |
1.7% |
0% |
1.3% |
0.3% |
|
|
| Agitation |
1.1% |
0.5% |
— |
|
|
|
|
|
| Tremor |
1.1% |
0.3% |
— |
|
|
|
|
|
| Anxiety |
— |
— |
— |
|
|
|
|
|
| Dizziness |
— |
— |
1.5% |
0% |
|
|
1% |
0.2% |
|
Gastrointestinal
|
|
|
|
|
|
|
|
|
| Constipation |
— |
|
1.1% |
0% |
|
|
|
|
| Nausea |
3.2% |
1.1% |
1.9% |
0% |
3.2% |
1.2% |
2% |
0.2% |
| Diarrhea |
1% |
0.3% |
— |
|
|
|
|
|
| Dry mouth |
1% |
0.3% |
— |
|
|
|
|
|
| Vomiting |
1% |
0.3% |
— |
|
|
|
|
|
| Flatulence |
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
|
|
|
|
| Asthenia |
1.6% |
0.4% |
1.9% |
0.4% |
|
|
1.8% |
0.2% |
| Abnormal ejaculation1
|
1.6% |
0% |
2.1% |
0% |
|
|
2.5% |
0.5% |
| Sweating |
1% |
0.3% |
— |
|
|
|
1.1% |
0.2% |
| Impotence1
|
— |
|
1.5% |
0% |
|
|
|
|
| Libido Decreased |
|
|
|
|
|
|
|
|
Commonly Observed Adverse Events
Major Depressive Disorder
Obsessive Compulsive Disorder
Panic Disorder
Generalized Anxiety Disorder
Incidence in Controlled Clinical Trials
Major Depressive Disorder
|
Body System |
Preferred Term |
Paroxetine (n = 421) |
Placebo (n = 421) |
|---|---|---|---|
| Body as a Whole |
Headache Asthenia |
18% 15% |
17% 6% |
| Cardiovascular |
Palpitation Vasodilation |
3% 3% |
1% 1% |
| Dermatologic |
Sweating Rash |
11% 2% |
2% 1% |
| Gastrointestinal |
Nausea Dry Mouth Constipation Diarrhea Decreased Appetite Flatulence Oropharynx Disorder2 Dyspepsia |
26% 18% 14% 12% 6% 4% 2% 2% |
9% 12% 9% 8% 2% 2% 0% 1% |
| Musculoskeletal |
Myopathy Myalgia Myasthenia |
2% 2% 1% |
1% 1% 0% |
| Nervous System |
Somnolence Dizziness Insomnia Tremor Nervousness Anxiety Paresthesia Libido Decreased Drugged Feeling Confusion |
23% 13% 13% 8% 5% 5% 4% 3% 2% 1% |
9% 6% 6% 2% 3% 3% 2% 0% 1% 0% |
| Respiration |
Yawn |
4% |
0% |
| Special Senses |
Blurred Vision Taste Perversion |
4% 2% |
1% 0% |
| Urogenital System |
Ejaculatory Disturbance3,4
Other Male Genital Disorders3,5 Urinary Frequency Urination Disorder6 Female Genital Disorders3,7 |
13% 10% 3% 3% 2% |
0% 0% 1% 0% 0% |
- Events reported by at least 1% of patients treated with paroxetine are included, except the following events which had an incidence on placebo ≥ paroxetine: Abdominal pain, agitation, back pain, chest pain, CNS stimulation, fever, increased appetite, myoclonus, pharyngitis, postural hypotension, respiratory disorder includes mostly “cold symptoms” or “URI”), trauma, and vomiting.
2. Includes mostly “lump in throat” and “tightness in throat.”
3. Percentage corrected for gender.
4. Mostly “ejaculatory delay.”
5. Includes “anorgasmia,” “erectile difficulties,” “delayed ejaculation/orgasm,” and “sexual dysfunction,” and “impotence.”
6. Includes mostly “difficulty with micturition” and “urinary hesitancy.”
7. Includes mostly “anorgasmia” and “difficulty reaching climax/orgasm.”
Obsessive Compulsive Disorder and Panic Disorder
|
Body System |
Preferred Term |
Obsessive Compulsive Disorder |
Panic Disorder | ||
|---|---|---|---|---|---|
| Paroxetine (n = 542) |
Placebo (n = 265) |
Paroxetine (n = 469) |
Placebo (n = 324) |
||
| Body as a Whole |
Asthenia |
22% |
14% |
14% |
5% |
| Abdominal Pain |
— |
— |
4% |
3% |
|
| Chest Pain |
3% |
2% |
— |
— |
|
| Back Pain |
— |
— |
3% |
2% |
|
| Chills |
2% |
1% |
2% |
1% |
|
| Trauma |
— |
— |
— |
— |
|
| Cardiovascular |
Vasodilation |
4% |
1% |
— |
— |
| Palpitation |
2% |
0% |
— |
— |
|
| Dermatologic |
Sweating |
9% |
3% |
14% |
6% |
| Rash |
3% |
2% |
— |
— |
|
| Gastrointestinal |
Nausea |
23% |
10% |
23% |
17% |
| Dry Mouth |
18% |
9% |
18% |
11% |
|
| Constipation |
16% |
6% |
8% |
5% |
|
| Diarrhea |
10% |
10% |
12% |
7% |
|
| Decreased Appetite |
9% |
3% |
7% |
3% |
|
| Dyspepsia |
— |
— |
— |
— |
|
| Flatulence |
— |
— |
— |
— |
|
| Increased Appetite |
4% |
3% |
2% |
1% |
|
| Vomiting |
— |
— |
— |
— |
|
| Musculoskeletal |
Myalgia |
— |
— |
— |
— |
| Nervous System |
Insomnia |
24% |
13% |
18% |
10% |
| Somnolence |
24% |
7% |
19% |
11% |
|
| Dizziness |
12% |
6% |
14% |
10% |
|
| Tremor |
11% |
1% |
9% |
1% |
|
| Nervousness |
9% |
8% |
— |
— |
|
| Libido Decreased |
7% |
4% |
9% |
1% |
|
| Agitation |
— |
— |
5% |
4% |
|
| Anxiety |
— |
— |
5% |
4% |
|
| Abnormal Dreams |
4% |
1% |
— |
— |
|
| Concentration Impaired |
3% |
2% |
— |
— |
|
| Depersonalization |
3% |
0% |
— |
— |
|
| Myoclonus |
3% |
0% |
3% |
2% |
|
| Amnesia |
2% |
1% |
— |
— |
|
| Respiratory System |
Rhinitis |
— |
— |
3% |
0% |
| Pharyngitis |
— |
— |
— |
— |
|
| Yawn |
— |
— |
— |
— |
|
| Special Senses |
Abnormal Vision |
4% |
2% |
— |
— |
| Taste Perversion |
2% |
0% |
— |
— |
|
| Urogenital System |
Abnormal Ejaculation2 |
23% |
1% |
21% |
1% |
| Dysmenorrhea |
— |
— |
— |
— |
|
| Female Genital Disorder2 |
3% |
0% |
9% |
1% |
|
| Impotence2
|
8% |
1% |
5% |
0% |
|
| Urinary Frequency |
3% |
1% |
2% |
0% |
|
| Urination Impaired |
3% |
0% |
— |
— |
|
| Urinary Tract Infection |
2% |
1% |
2% |
1% |
|
- Events reported by at least 2% of OCD and panic disorder in patients treated with paroxetine are included, except the following events which had an incidence on placebo ≥ paroxetine: [OCD]: Abdominal pain, agitation, anxiety, back pain, cough increased, depression, headache, hyperkinesia, infection, paresthesia, pharyngitis, respiratory disorder, rhinitis, and sinusitis. [panic disorder]: Abnormal dreams, abnormal vision, chest pain, cough increased, depersonalization, depression, dysmenorrhea, dyspepsia, flu syndrome, headache, infection, myalgia, nervousness, palpitation, paresthesia, pharyngitis, rash, respiratory disorder, sinusitis, taste perversion, trauma, urination impaired, and vasodilation.
- Percentage corrected for gender.
Generalized Anxiety Disorder
|
Body System |
Preferred Term |
Generalized Anxiety Disorder |
|
|---|---|---|---|
| Paroxetine (n = 735) |
Placebo (n = 529) |
||
| Body as a Whole
|
Asthenia
|
14% |
6% |
| Headache |
17% |
14% |
|
| Infection |
6% |
3% |
|
| Abdominal Pain |
|
|
|
| Trauma
|
|
|
|
| Cardiovascular
|
Vasodilation |
3% |
1% |
| Dermatologic |
Sweating |
6% |
2% |
| Gastrointestinal
|
Nausea |
20% |
5% |
| Dry Mouth |
11% |
5% |
|
| Constipation |
10% |
2% |
|
| Diarrhea |
9% |
7% |
|
| Decreased Appetite |
5% |
1% |
|
| Vomiting |
3% |
2% |
|
| Dyspepsia |
— |
— |
|
| Nervous System
|
Insomnia |
11% |
8% |
| Somnolence |
15% |
5% |
|
| Dizziness |
6% |
5% |
|
| Tremor |
5% |
1% |
|
| Nervousness |
4% |
3% |
|
| Libido Decreased |
9% |
2% |
|
| Abnormal Dreams |
|
|
|
| Respiratory System
|
Respiratory Disorder |
7% |
5% |
| Sinusitis |
4% |
3% |
|
| Yawn |
4% |
— |
|
| Special Senses
|
Abnormal Vision |
2% |
1% |
| Urogenital System
|
Abnormal Ejaculation2
|
25% |
2% |
| Female Genital Disorder2
|
4% |
1% |
|
| Impotence2
|
4% |
3% |
|
- Events reported by at least 2% of GAD in patients treated with paroxetine are included, except the following events which
had an incidence on placebo ≥ paroxetine [GAD]: Abdominal pain, back pain, trauma, dyspepsia, myalgia, and pharyngitis.
Dose Dependency of Adverse Events
|
Body System/Preferred Term |
Placebo | Paroxetine | |||
|---|---|---|---|---|---|
| n = 51 | 10 mg n = 102 |
20 mg n = 104 |
30 mg n = 101 |
40 mg n = 102 |
|
|
* Rule for including adverse events in table: Incidence at least 5% for 1 of paroxetine groups and ≥ twice the placebo incidence for at least 1 paroxetine group. |
|||||
|
Body as a Whole
|
|
|
|
|
|
| Asthenia |
0% |
2.9% |
10.6% |
13.9% |
12.7% |
|
Dermatology
|
|
|
|
|
|
| Sweating |
2% |
1% |
6.7% |
8.9% |
11.8% |
|
Gastrointestinal
|
|
|
|
|
|
| Constipation |
5.9% |
4.9% |
7.7% |
9.9% |
12.7% |
| Decreased Appetite |
2% |
2% |
5.8% |
4% |
4.9% |
| Diarrhea |
7.8% |
9.8% |
19.2% |
7.9% |
14.7% |
| Dry Mouth |
2% |
10.8% |
18.3% |
15.8% |
20.6% |
| Nausea |
13.7% |
14.7% |
26.9% |
34.7% |
36.3% |
|
Nervous System
|
|
|
|
|
|
| Anxiety |
0% |
2% |
5.8% |
5.9% |
5.9% |
| Dizziness |
3.9% |
6.9% |
6.7% |
8.9% |
12.7% |
| Nervousness |
0% |
5.9% |
5.8% |
4.0% |
2.9% |
| Paresthesia |
0% |
2.9% |
1% |
5% |
5.9% |
| Somnolence |
7.8% |
12.7% |
18.3% |
20.8% |
21.6% |
| Tremor |
0% |
0% |
7.7% |
7.9% |
14.7% |
|
Special Senses
|
|
|
|
|
|
| Blurred Vision |
2% |
2.9% |
2.9% |
2% |
7.8% |
|
Urogenital System
|
|
|
|
|
|
| Abnormal Ejaculation |
0% |
5.8% |
6.5% |
10.6% |
13% |
| Impotence |
0% |
1.9% |
4.3% |
6.4% |
1.9% |
| Male Genital Disorders |
0% |
3.8% |
8.7% |
6.4% |
3.7% |
Adaptation to Certain Adverse Events
Male and Female Sexual Dysfunction With SSRIs
| |
Paroxetine | Placebo |
|---|---|---|
|
n (males)
|
1446
|
1042
|
| Decreased Libido |
6-15% |
0-5% |
| Ejaculatory Disturbance |
13-28% |
0-2% |
| Impotence |
2-9% |
0-3% |
|
n (females)
|
1822
|
1340
|
| Decreased Libido |
0-9% |
0-2% |
| Orgasmic Disturbance |
2-9% |
0-1% |
Weight and Vital Sign Changes
ECG Changes
Liver Function Tests
Hallucinations
Other Events Observed During the Premarketing Evaluation of Paroxetine
PRECAUTIONS
Body as a Whole
Infrequent:rare:
Cardiovascular System
Frequent:infrequent:rare:
Digestive System
Infrequent:rare:
Endocrine System
Rare:
Hemic and Lymphatic Systems
Infrequent:rare:
Metabolic and Nutritional
Frequent:infrequent:rare:
Musculoskeletal System
Frequent:infrequent:rare:
Nervous System
Frequent:infrequent:rare:
Respiratory System
Infrequent: rare:
Skin and Appendages
Frequent:infrequent:rare:
Special Senses
Frequent:infrequent:rare:
Urogenital System
Infrequent:rare:
Postmarketing Reports
Voluntary reports of adverse events in patients taking paroxetine that have been received since market introduction and not listed above that may have no causal relationship with the drug include acute pancreatitis, elevated liver function tests (the most severe cases were deaths due to liver necrosis, and grossly elevated transaminases associated with severe liver dysfunction), Guillain-Barré syndrome, toxic epidermal necrolysis, priapism, syndrome of inappropriate ADH secretion, symptoms suggestive of prolactinemia and galactorrhea; extrapyramidal symptoms which have included akathisia, bradykinesia, cogwheel rigidity, dystonia, hypertonia, oculogyric crisis which has been associated with concomitant use of pimozide; tremor and trismus; status epilepticus, acute renal failure, pulmonary hypertension, allergic alveolitis, anaphylaxis, eclampsia, laryngismus, optic neuritis, porphyria, ventricular fibrillation, ventricular tachycardia (including torsade de pointes), thrombocytopenia, hemolytic anemia, events related to impaired hematopoiesis (including aplastic anemia, pancytopenia, bone marrow aplasia, and agranulocytosis), and vasculitic syndromes (such as Henoch-Schönlein purpura). There has been a case report of an elevated phenytoin level after 4 weeks of paroxetine and phenytoin coadministration. There has been a case report of severe hypotension when paroxetine was added to chronic metoprolol treatment.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class
Physical and Psychologic Dependence
Paroxetine has not been systematically studied in animals or humans for its potential for abuse, tolerance or physical dependence. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for history of drug abuse, and such patients should be observed closely for signs of misuse or abuse of paroxetine (e.g., development of tolerance, incrementations of dose, drug-seeking behavior).
OVERDOSAGE
Human Experience
Overdosage Management
PRECAUTIONS—Drugs Metabolized by Cytochrome CYP2D6
Physicians' Desk Reference
PAROXETINE DOSAGE AND ADMINISTRATION
Major Depressive Disorder
Usual Initial Dosage
Maintenance Therapy
Obsessive Compulsive Disorder
Usual Initial Dosage
Maintenance Therapy
CLINICAL PHARMACOLOGY—Clinical Trials
Panic Disorder
Usual Initial Dosage
Maintenance Therapy
CLINICAL PHARMACOLOGY—Clinical Trials
Generalized Anxiety Disorder
Usual Initial Dosage
Maintenance Therapy
CLINICAL PHARMACOLOGY—Clinical Trials
Special Populations
Treatment of Pregnant Women During the Third Trimester
WARNINGS
Dosage for Elderly or Debilitated Patients, and Patients With Severe Renal or Hepatic Impairment
Switching Patients to or From a Monoamine Oxidase Inhibitor
Discontinuation of Treatment With Paroxetine Tablets
PRECAUTIONS
HOW SUPPLIED
Paroxetine Tablets USP, 10 mg
Paroxetine Tablets USP, 20 mg
Paroxetine Tablets USP, 30 mg
Paroxetine Tablets USP, 40 mg
They are supplied by State of Florida DOH Central Pharmacy as follows:
| NDC | Strength | Quantity/Form | Color | Source Prod. Code |
| 53808-0749-1 | 20 mg | 30 Tablets in a Blister Pack | PINK | 65862-155 |
| 53808-0752-1 | 30 mg | 30 Tablets in a Blister Pack | BLUE | 65862-156 |
| 53808-0756-1 | 40 mg | 30 Tablets in a Blister Pack | PINK | 65862-157 |
Store at
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
MEDICATION GUIDE
Antidepressant Medicines, Depression and Other Serious Mental Illnesses,
and Suicidal Thoughts or Actions
Talk to your, or your family member’s, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
20mg Label
_0a580de2.jpg)
30mg Label
_0a580de2.jpg)
40mg Label
_0a580de2.jpg)
ParoxetinePAROXETINE HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ParoxetinePAROXETINE HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ParoxetinePAROXETINE HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||