Pedi-Dri
PEDI-DRI TOPICAL POWDER Nystatin Topical Powder USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- PEDI-DRI DESCRIPTION
- CLINICAL PHARMACOLOGY
- PEDI-DRI INDICATIONS AND USAGE
- PEDI-DRI CONTRAINDICATIONS
- PRECAUTIONS
- PEDI-DRI ADVERSE REACTIONS
- PEDI-DRI DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 56.7 g Bottle Label
FULL PRESCRIBING INFORMATION
Rx only
For topical use only.
Not for ophthalmic use.
PEDI-DRI DESCRIPTION
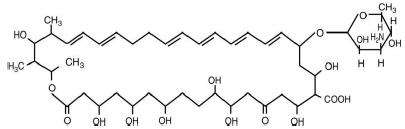
PEDI-DRI®, Nystatin Topical Powder USP is for dermatologic use.
PEDI-DRI® provides, in each gram, 100,000 USP nystatin units dispersed in talc.
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces nursei.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Nystatin is not absorbed from intact skin or mucous membrane.
Microbiology
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes.
Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida (c. tropicalis, C. guilliermondi, C. krusei, and C. stalltoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
PEDI-DRI INDICATIONS AND USAGE
PEDI-DRI® is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by Candida albicans and other susceptible Candida species.
This preparation is not indicated for systemic, oral, intravaginal or ophthalmic use.
PEDI-DRI CONTRAINDICATIONS
PEDI-DRI® is contraindicated in patients with a history of hypersensitivity to any of its components.
PRECAUTIONS
General
Nystatin Topical Powder should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
INFORMATION FOR THE PATIENT
Patients using this medication should receive the following information and instructions:
- The patient should be instructed to use this medication as directed (including the replacement of missed doses). This medication is not for any disorder other than that for which it is prescribed.
- Even if symptomatic relief occurs within the first few days of treatment, the patient should be advised not to interrupt or discontinue therapy until the prescribed course of treatment is completed.
Laboratory Tests
If there is a lack of therapeutic response, KOH smears, cultures, or other diagnostic methods should be repeated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate the carcinogenic potential of nystatin. No studies have been performed to determine the mutagenicity of nystatin or its effects on male or female fertility.
Pregnancy
Teratogenic Effects
Category C
Animal reproduction studies have not been conducted with any nystatin topical preparation. It also is not known whether these preparations can cause fetal harm when used by a pregnant woman or can affect reproductive capacity. Nystatin topical preparations should be prescribed for a pregnant woman only if the potential benefit to the mother outweighs the potential risk to the fetus.
Nursing Mothers
It is not known whether nystatin is excreted in human milk. Caution should be exercised when nystatin is prescribed for a nursing woman.
Pediatric Use
Safety and effectiveness have been established in the pediatric population from birth to 16 years.
(See DOSAGE AND ADMINISTRATION).
PEDI-DRI ADVERSE REACTIONS
The frequency of adverse events reported in patients using nystatin topical preparations is less that 0.1%. The more common events that were reported include allergic reactions, burning, itching, rash, eczema, and pain on application (see PRECAUTIONS: General.)
PEDI-DRI DOSAGE AND ADMINISTRATION
Very moist lesions are best treated with the topical dusting powder.
Adults and Pediatric Patients (Neonates and Older)
Apply to candidal lesions two or three times daily until healing is complete. For fungal infection of the feet caused by Candida species, the powder should be dusted on the feet, as well as, in all foot wear.
PEDI-DRI® does not stain skin or mucous membranes and provides a simple, convenient means of treatment.
HOW SUPPLIED
PEDI-DRI®, Nystatin Topical Powder USP, supplied as 2 oz. of powder in a 6 oz. plastic squeeze bottle.
PEDI-DRI® TOPICAL POWDER
Nystatin Topical Powder USP 56.7g (2oz.)
NDC 0884-0396-02
Keep tightly closed.
Store at controlled room temperature 15° - 30°C (59° - 86°F); avoid excessive heat 40°C; 104°F).
Manufactured for:
PEDINOL PHARMACAL INC.
Farmingdale, NY 11735
(09-01)
Each gram contains 100,000 USP nystatin units dispersed in talc.
FOR TOPICAL USE ONLY.
Not for Ophthalmic Use.
Usual Dosage: Apply to affected area 2 or 3 times daily.
See insert – Keep tightly closed
Store at controlled room temperature 15°-30°C (59° - 86°F); avoid excessive heat (40°C; 104°F).
Manufactured for:
PEDINOL PHARMACAL INC.
Farmingdale, NY 11735
2122662 (09-01)
PRINCIPAL DISPLAY PANEL - 56.7 g Bottle Label
NDC 0884-0396-02
PEDI-DRI
®
TOPICAL POWDER
Nystatin Topical Powder USP
100,000 USP units per gram
Rx only
Net Wt: 56.7g (2 oz.)
PEDiNOL
PHARMACAL INC

Pedi-DriNystatin POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||