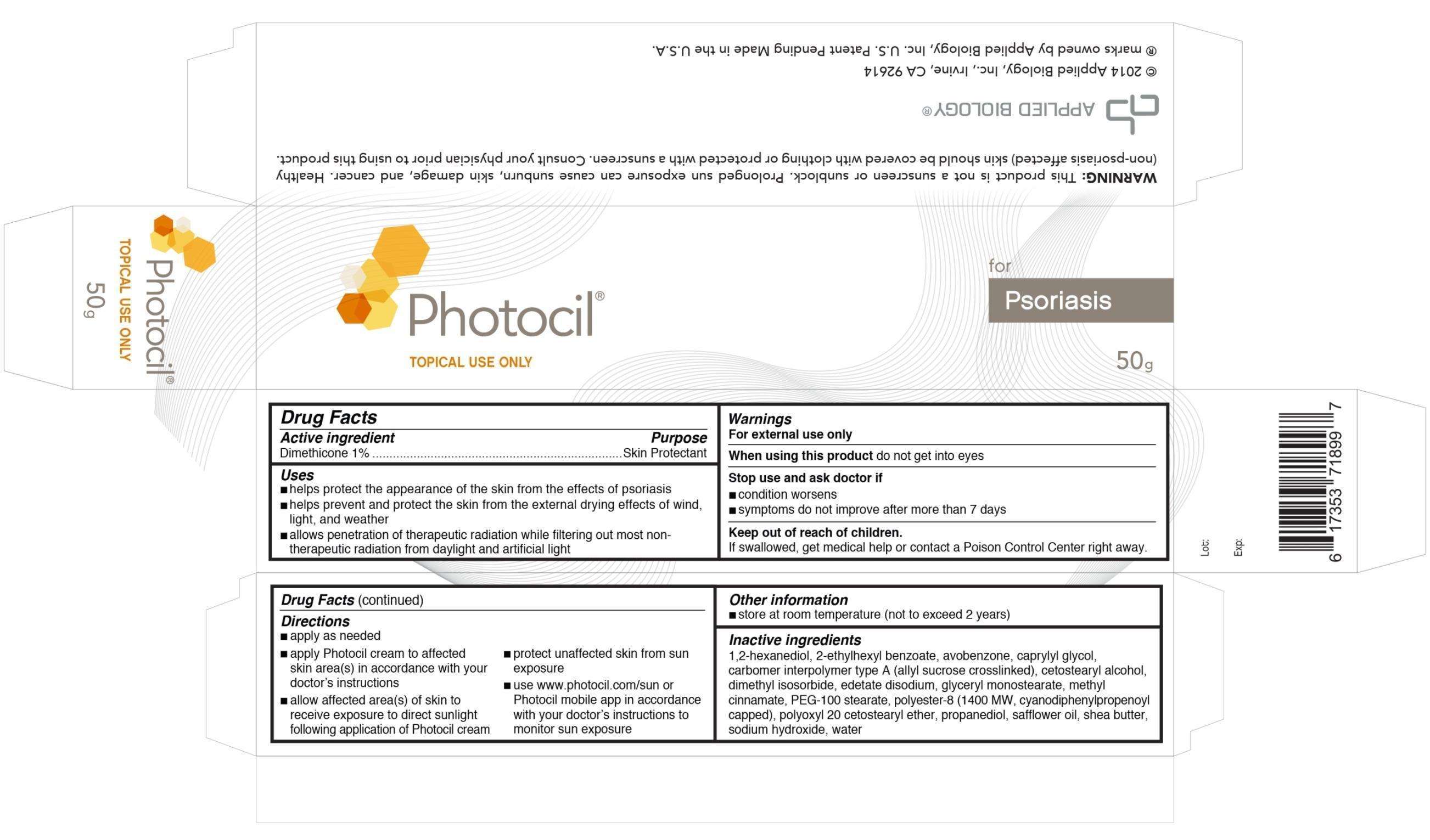
Photocil
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Photocil Uses
- Warnings
- Directions
- Photocil Other information
- Inactive ingredients
- Carton Label
FULL PRESCRIBING INFORMATION
Active Ingredient
Dimethicone 1%
Purpose
Skin Protectant
Photocil Uses
- helps protect the appearance of the skin from the effects of psoriasis
- helps prevent and protect the skin from the external drying effects of wind, light and weather
- allows penetration of therapeutic radiation while filtering out most non-therapeutic radiation from daylight and artificial light
Warnings
For external use only
When using this product do not get into eyes
Stop use and ask doctor if
- condition worsens
- symptoms do not improve after more than 7 days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply as needed
- apply Photocil cream to affected skin area(s) in accordance with your doctor's instructions
- allow affected area(s) of skin to receive exposure to direct sunlight following application of Photocil cream
- protect unaffected skin from sun exposure
- use www.photocil.com/sun or Photocil mobile app in accordance with your doctor's instructions to monitor sun exposure
Photocil Other information
- store at room temperature (not to exceed 2 years)
Inactive ingredients
1,2-hexanediol, 2-ethylhexyl benzoate, avobenzone, caprylyl glycol, carbomer interpolymer type a (allyl sucrose crosslinked), cetostearyl alcohol,
dimethyl isosorbide, edetate disodium, glyceryl monostearate, methyl cinnamate, PEG-100 stearate, polyester-8 (1400MW, cyanodiphenylpropenoyl capped), polyoxyl 20 cetostearyl ether, propanediol, safflower oil, shea butter, sodium hydroxide, water
Carton Label
PhotocilDimethicone CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!