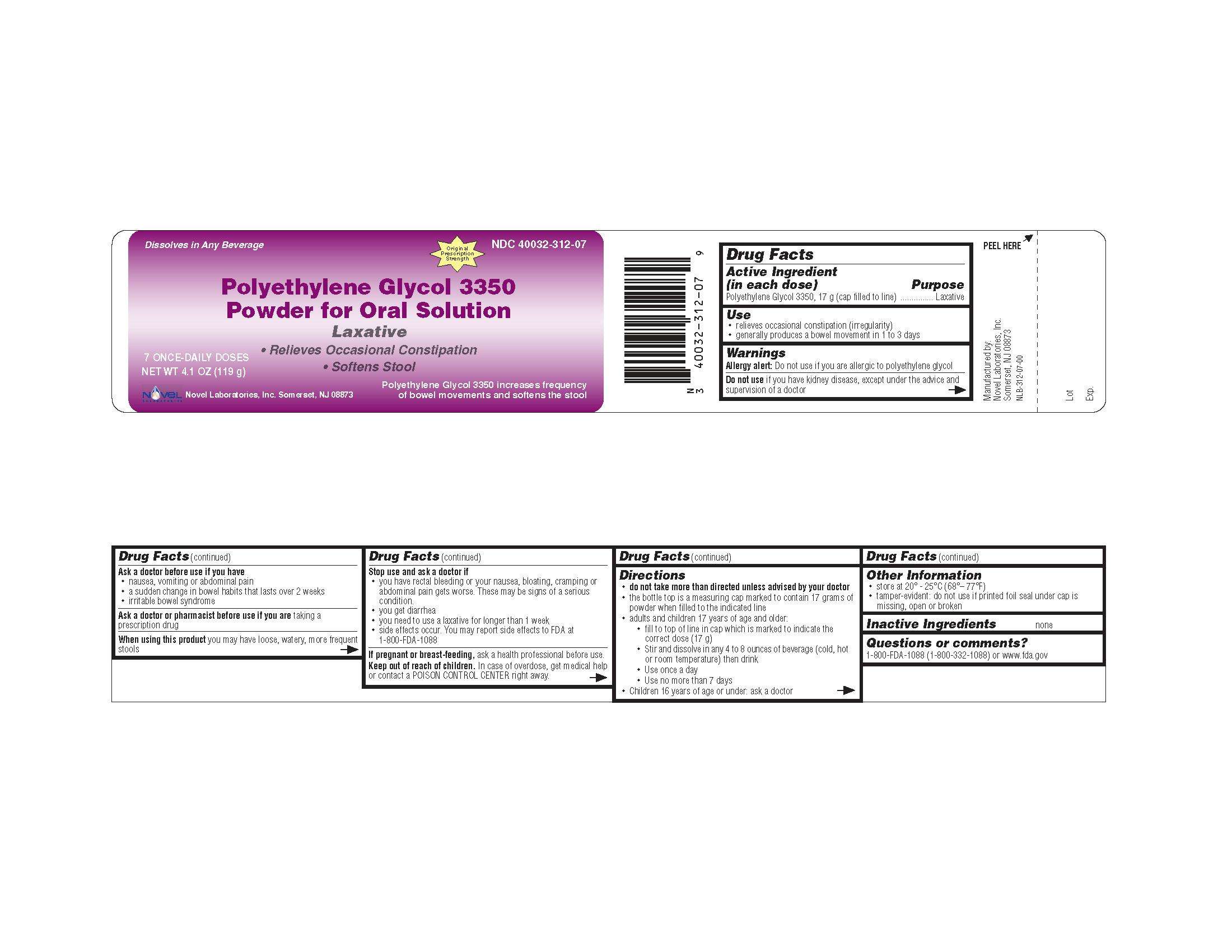
Polyethylene Glycol-3350
Novel Laboratories, Inc.
Novel Laboratories, Inc.
Polyethylene Glycol 3350, Powder for Oral Solution
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient (in each dose)..........................................Purpose
Polyethylene Glycol 3350, 17 g (cap filled to line).....................Laxative
Use
-
relieves occasional constipation (irregularity)
-
generally produces a bowel movement in 1 to 3 days
Allergy alert: Do not use if you are allergic to polyethylene glycol
Do not use if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
-
nausea, vomiting or abdominal pain
-
a sudden change in bowel habits that lasts over 2 weeks
-
irritable bowel syndrome
Ask a doctor or pharmacist before use if you are taking a prescription drug
When using this product you may have loose, watery, more frequent stools
Stop use and ask a doctor if
-
you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
-
you get diarrhea
-
you need to use a laxative for longer than 1 week
-
side effects occur. You may report side effects to FDA at 1-800-FDA-1088
Drug Facts (continued)
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a POISON CONTROL CENTER right away.
Directions
-
do not take more than directed unless advised by your doctor
-
the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line.
-
adults and children 17 years of age and older:
-
fill to top of line in cap which is marked to indicate the correct dose (17 g)
-
stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
-
use once a day
-
use no more than 7 days
-
-
children 16 years of age or under: ask a doctor
Other Information
-
store at 20° - 25°C (68°– 77°F)
-
tamper-evident: do not use if printed foil seal under cap is missing, open or broken
Inactive Ingredients none
Questions or comments?
1-800-FDA-1088 (1-800-332-1088) or www.fda.gov


Polyethylene Glycol-3350Polyethylene Glycol-3350 POWDER, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||