Pomalyst
HIGHLIGHTS OF PRESCRIBING INFORMATION BOXED WARNING WARNING: EMBRYO-FETAL TOXICITY and VENOUS THROMBOEMBOLISM See full prescribing information for complete boxed warning EMBRYO-FETAL TOXICITY POMALYST is contraindicated in pregnancy. POMALYST is a thalidomide analogue. Thalidomide is a known human teratogen that causes severe life-threatening birth defects (4, 5.1, 8.1). For females of reproductive potential: Exclude pregnancy before start of treatment. Prevent pregnancy during treatment by the use of 2 reliable methods of contraception (5.1, 8.6). POMALYST is available only through a restricted program called POMALYST REMS™ (5.2). VENOUS THROMBOEMBOLISM Deep venous thrombosis (DVT) and pulmonary embolism (PE) occur in patients with multiple myeloma treated with POMALYST (5.3). RECENT MAJOR CHANGESDosage and Administration (2.3) 03/14Warnings and Precautions (5.9) 05/14INDICATIONS AND USAGEPOMALYST is a thalidomide analogue indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate. Clinical benefit, such as improvement in survival or symptoms, has not been verified. DOSAGE AND ADMINISTRATION4 mg per day taken orally on Days 1-21 of repeated 28-day cycles until disease progressionDOSAGE FORMS AND STRENGTHSCapsules: 1 mg, 2 mg, 3 mg, and 4 mg (3) CONTRAINDICATIONS Pregnancy (4) WARNINGS AND PRECAUTIONS Hematologic Toxicity: Neutropenia was the most frequently reported Grade 3/4 adverse event. Monitor patients for hematologic toxicities, especially neutropenia (5.4). Tumor Lysis Syndrome (TLS): Monitor patients at risk of TLS (i.e., those with high tumor burden) and take appropriate precautions (5.9). Side EffectsMost common adverse reactions (≥30%) included fatigue and asthenia, neutropenia, anemia, constipation, nausea, diarrhea, dyspnea, upper-respiratory tract infections, back pain and pyrexia (6.1). To report SUSPECTED ADVERSE REACTIONS, contact Celgene Corporation at 1-888-423-5436 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Strong CYP1A2 Inhibitors: Avoid the use of strong CYP1A2 inhibitors unless medically necessary (2.3, 7.1, 12.3). USE IN SPECIFIC POPULATIONS Nursing Mothers: Discontinue drug or nursing taking into consideration importance of drug to mother (8.3). Avoid POMALYST in patients with serum creatinine >3.0 mg/dL (8.7).
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: EMBRYO-FETAL TOXICITY and VENOUS THROMBOEMBOLISM
- RECENT MAJOR CHANGES
- 1 POMALYST INDICATIONS AND USAGE
- 2 POMALYST DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 POMALYST CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 POMALYST ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 POMALYST DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
WARNING: EMBRYO-FETAL TOXICITY and VENOUS THROMBOEMBOLISM
Embryo-Fetal Toxicity
- POMALYST is contraindicated in pregnancy. POMALYST is a thalidomide analogue. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting POMALYST treatment.
- Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after stopping POMALYST treatment [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.6)].
POMALYST is only available through a restricted distribution program called POMALYST REMS™ [see Warnings and Precautions (5.2)].
Venous Thromboembolism
- Deep venous thrombosis (DVT) and pulmonary embolism (PE) occur in patients with multiple myeloma treated with POMALYST. Prophylactic anti-thrombotic measures were employed in the clinical trial. Consider prophylactic measures after assessing an individual patient’s underlying risk factors [see Warnings and Precautions (5.3)].
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
1.1 Multiple Myeloma
POMALYST is indicated for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 days of completion of the last therapy. Approval is based on response rate [see Clinical Studies (14.1)]. Clinical benefit, such as improvement in survival or symptoms, has not been verified.
2 DOSAGE AND ADMINISTRATION
2.1 Multiple Myeloma
Females of reproductive potential must have negative pregnancy testing and use contraception methods before initiating POMALYST [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
The recommended starting dose of POMALYST is 4 mg once daily orally on Days 1-21 of repeated 28-day cycles until disease progression. POMALYST may be given in combination with dexamethasone [see Clinical Studies (14.1)].
POMALYST may be taken with water. Inform patients not to break, chew, or open the capsules. POMALYST should be taken without food (at least 2 hours before or 2 hours after a meal).
2.2 Dose Adjustments for Toxicities
| ANC, absolute neutrophil count. | |
| Toxicity | Dose Modification |
Neutropenia
|
|
|
|
Thrombocytopenia
|
|
|
|
For other Grade 3 or 4 toxicities, hold treatment and restart treatment at 1 mg less than the previous dose when toxicity has resolved to less than or equal to Grade 2 at the physician’s discretion.
To initiate a new cycle of POMALYST, the neutrophil count must be at least 500 per mcL and the platelet count must be at least 50,000 per mcL. If toxicities occur after dose reductions to 1 mg, then discontinue POMALYST.
Avoid co-administration of strong inhibitors of CYP1A2. If necessary to co-administer strong inhibitors of CYP1A2 in the presence of strong inhibitors of CYP3A4 and P-gp, reduce POMALYST dose by 50%. No clinical efficacy or safety data exist [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
POMALYST is available in the following capsule strengths:
1 mg: Dark blue opaque cap and yellow opaque body, imprinted “POML” on the cap in white ink and “1 mg” on the body in black ink
2 mg: Dark blue opaque cap and orange opaque body, imprinted “POML” on the cap and “2 mg” on the body in white ink
3 mg: Dark blue opaque cap and green opaque body, imprinted “POML” on the cap and “3 mg” on the body in white ink
4 mg: Dark blue opaque cap and blue opaque body, imprinted “POML” on the cap and “4 mg” on the body in white ink
4 CONTRAINDICATIONS
Pregnancy
POMALYST can cause fetal harm when administered to a pregnant female [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)]. POMALYST is contraindicated in females who are pregnant. Pomalidomide is a thalidomide analogue and is teratogenic in both rats and rabbits when administered during the period of organogenesis. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
POMALYST is a thalidomide analogue and is contraindicated for use during pregnancy. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death [see Use in Specific Populations (8.1)]. POMALYST is only available through the POMALYST REMS program [see Warnings and Precautions (5.2)].
Females of Reproductive Potential
Females of reproductive potential must avoid pregnancy while taking POMALYST and for at least 4 weeks after completing therapy.
Females must commit either to abstain continuously from heterosexual sexual intercourse or to use 2 methods of reliable birth control, beginning 4 weeks prior to initiating treatment with POMALYST, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of POMALYST therapy.
Two negative pregnancy tests must be obtained prior to initiating therapy. The first test should be performed within 10-14 days and the second test within 24 hours prior to prescribing POMALYST therapy and then weekly during the first month, then monthly thereafter in women with regular menstrual cycles, or every 2 weeks in women with irregular menstrual cycles [see Use in Specific Populations (8.6)].
Males
Pomalidomide is present in the semen of patients receiving the drug. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 28 days after discontinuing POMALYST, even if they have undergone a successful vasectomy. Male patients taking POMALYST must not donate sperm [see Use in Specific Populations (8.6)].
Blood Donation
Patients must not donate blood during treatment with POMALYST and for 1 month following discontinuation of the drug because the blood might be given to a pregnant female patient whose fetus must not be exposed to POMALYST.
5.2 POMALYST REMS Program
Because of the embryo-fetal risk [see Warnings and Precautions (5.1)], POMALYST is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called “POMALYST REMS.”
Required components of the POMALYST REMS program include the following:
- Prescribers must be certified with the POMALYST REMS program by enrolling and complying with the REMS requirements.
- Patients must sign a Patient-Prescriber agreement form and comply with the REMS requirements. In particular, female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements [see Use in Specific Populations (8.6)] and males must comply with contraception requirements [see Use in Specific Populations (8.6)].
- Pharmacies must be certified with the POMALYST REMS program, must only dispense to patients who are authorized to receive POMALYST and comply with REMS requirements.
Further information about the POMALYST REMS program is available at www.CelgeneRiskManagement.com or by telephone at 1-888-423-5436.
5.3 Venous Thromboembolism
Patients receiving POMALYST have developed venous thromboembolic events (VTEs) (venous thromboembolism) reported as serious adverse reactions. In the trial, all patients were required to receive prophylaxis or anti-thrombotic treatment; 81% used aspirin, 16% warfarin, 21% heparin, and 3% clopidogrel. The rate of deep vein thrombosis or pulmonary embolism was 3%. Consider anti-coagulation prophylaxis after an assessment of each patient’s underlying risk factors.
5.4 Hematologic Toxicity
Neutropenia was the most frequently reported Grade 3/4 adverse reaction, followed by anemia and thrombocytopenia. Neutropenia of any grade was reported in 50% of patients in the trial. The rate of Grade 3/4 neutropenia was 43%. The rate of febrile neutropenia was 3%.
Monitor patients for hematologic toxicities, especially neutropenia. Monitor complete blood counts weekly for the first 8 weeks and monthly thereafter. Patients may require dose interruption and/or modification [see Dosage and Administration (2.2)].
5.5 Hypersensitivity Reactions
Patients with a prior history of serious hypersensitivity associated with thalidomide or lenalidomide were excluded from studies and may be at higher risk of hypersensitivity.
5.6 Dizziness and Confusional State
In the trial, 18% of patients experienced dizziness and 12% of patients experienced a confusional state; 1% of patients experienced Grade 3/4 dizziness, and 3% of patients experienced Grade 3/4 confusional state. Instruct patients to avoid situations where dizziness or confusional state may be a problem and to not take other medications that may cause dizziness or confusional state without adequate medical advice.
5.7 Neuropathy
In the trial, 18% of patients experienced neuropathy, with approximately 9% of the patients experiencing peripheral neuropathy. There were no cases of Grade 3 or higher neuropathy adverse reactions reported.
5.8 Risk of Second Primary Malignancies
Cases of acute myelogenous leukemia have been reported in patients receiving POMALYST as an investigational therapy outside of multiple myeloma.
Tumor lysis syndrome (TLS) may occur in patients treated with pomalidomide. Patients at risk for TLS are those with high tumor burden prior to treatment. These patients should be monitored closely and appropriate precautions taken.
6 ADVERSE REACTIONS
The following adverse reactions are described in detail in other labeling sections:
- Fetal Risk [see Boxed Warnings, Warnings and Precautions (5.1, 5.2)]
- Venous Thromboembolism [see Boxed Warnings, Warnings and Precautions (5.3)]
- Hematologic Toxicity [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- Dizziness and Confusional State [see Warnings and Precautions (5.6)]
- Neuropathy [see Warnings and Precautions (5.7)]
- Risk of Second Primary Malignancies [see Warnings and Precautions (5.8)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience in Multiple Myeloma
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Clinical Trial 1, data were evaluated from 219 patients (safety population) who received treatment with POMALYST + Low-dose Dexamethasone (Low-dose Dex) (112 patients) or POMALYST alone (107 patients). Median number of treatment cycles was 5. Sixty-three percent of patients in the study had a dose interruption of either drug due to adverse reactions. Thirty-seven percent of patients in the study had a dose reduction of either drug due to adverse reactions. The discontinuation rate due to treatment-related adverse reaction was 3%.
Tables 2, 3, and 4 summarize all treatment-emergent adverse reactions reported for the POMALYST + Low-dose Dex and POMALYST alone groups regardless of attribution of relatedness to pomalidomide. In the absence of a randomized comparator arm, it is often not possible to distinguish adverse events that are drug related and those that reflect the patient’s underlying disease.
| aPOMALYST alone arm includes all patients randomized to the pomalidomide alone arm who took study drug; 61 of the 107 patients had dexamethasone added during the treatment period. | ||
| Trial 1 | ||
|
POMALYSTa (n=107) |
POMALYST + Low-dose Dex (n=112) |
|
| System Organ Class/Preferred Term | n (%) | n (%) |
| Patients With at Least One Treatment- Emergent Adverse Reaction |
107 (100) | 112 (100) |
|
General disorders and administration site conditions |
||
| Fatigue and asthenia | 59 (55) | 70 (63) |
| Pyrexia | 20 (19) | 34 (30) |
| Edema peripheral | 25 (23) | 18 (16) |
| Chills | 10 (9) | 12 (11) |
| Pain | 6 (6) | 5 (5) |
| Blood and lymphatic system disorders | ||
| Neutropenia | 56 (52) | 53 (47) |
| Anemia | 41 (38) | 44 (39) |
| Thrombocytopenia | 27 (25) | 26 (23) |
| Leukopenia | 12 (11) | 20 (18) |
| Lymphopenia | 4 (4) | 17 (15) |
| Gastrointestinal disorders | ||
| Constipation | 38 (36) | 39 (35) |
| Diarrhea | 36 (34) | 37 (33) |
| Nausea | 38 (36) | 25 (22) |
| Vomiting | 15 (14) | 15 (13) |
| Infections and infestations | ||
| Pneumonia | 25 (23) | 32 (29) |
| Upper respiratory tract infection | 34 (32) | 28 (25) |
| Urinary tract infection | 8 (8) | 18 (16) |
|
Musculoskeletal and connective tissue disorders |
||
| Back pain | 34 (32) | 34 (30) |
| Musculoskeletal chest pain | 23 (22) | 22 (20) |
| Muscle spasms | 20 (19) | 21 (19) |
| Arthralgia | 17 (16) | 17 (15) |
| Musculoskeletal pain | 12 (11) | 17 (15) |
| Pain in extremity | 5 (5) | 16 (14) |
| Muscular weakness | 13 (12) | 13 (12) |
| Bone pain | 13 (12) | 5 (5) |
|
Respiratory, thoracic, and mediastinal disorders |
||
| Dyspnea | 36 (34) | 50 (45) |
| Cough | 15 (14) | 23 (21) |
| Epistaxis | 16 (15) | 12 (11) |
| Metabolism and nutritional disorders | ||
| Decreased appetite | 23 (22) | 20 (18) |
| Hyperglycemia | 13 (12) | 17 (15) |
| Hyponatremia | 11 (10) | 14 (13) |
| Hypercalcemia | 22 (21) | 13 (12) |
| Hypocalcemia | 6 (6) | 13 (12) |
| Hypokalemia | 11 (10) | 12 (11) |
| Skin and subcutaneous tissue disorders | ||
| Hyperhidrosis | 6 (6) | 18 (16) |
| Rash | 23 (22) | 18 (16) |
| Night sweats | 5 (5) | 14 (13) |
| Dry skin | 10 (9) | 12 (11) |
| Pruritus | 16 (15) | 12 (11) |
| Nervous system disorders | ||
| Dizziness | 21 (20) | 19 (17) |
| Tremor | 10 (9) | 14 (13) |
| Headache | 14 (13) | 9 (8) |
| Neuropathy peripheral | 11 (10) | 8 (7) |
| Investigations | ||
| Blood creatinine increased | 16 (15) | 12 (11) |
| Weight increased | 1 (1) | 12 (11) |
| Weight decreased | 15 (14) | 9 (8) |
| Psychiatric disorders | ||
| Insomnia | 7 (7) | 16 (14) |
| Confusional state | 11 (10) | 15 (13) |
| Anxiety | 12 (11) | 8 (7) |
| Renal and urinary disorders | ||
| Renal failure | 16 (15) | 11 (10) |
| a POMALYST alone arm includes all patients randomized to the pomalidomide alone arm who took study drug; 61 of the 107 patients had dexamethasone added during the treatment period. | ||
| Trial 1 | ||
|
POMALYSTa (n=107) |
POMALYST + Low-dose Dex (n=112) |
|
| System Organ Class/Preferred Term | n (%) | n (%) |
| Patients With at Least One Treatment- Emergent NCI CTC Grade 3 or 4 Adverse Reaction |
96 (90) | 99 (88) |
| Blood and lymphatic system disorders | ||
| Neutropenia | 50 (47) | 43 (38) |
| Anemia | 24 (22) | 23 (21) |
| Thrombocytopenia | 24 (22) | 21 (19) |
| Leukopenia | 6 (6) | 11 (10) |
| Lymphopenia | 2 (2) | 8 (7) |
| Infections and infestations | ||
| Pneumonia | 17 (16) | 26 (23) |
| Urinary tract infection | 2 (2) | 9 (8) |
| Sepsis | 6 (6) | 3 (3) |
| Metabolism and nutritional disorders | ||
| Hypercalcemia | 10 (9) | 1 (1) |
|
General disorders and administration site conditions |
||
| Fatigue and asthenia | 12 (11) | 14 (13) |
| Investigations | ||
| Blood creatinine increased | 6 (6) | 3 (3) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dyspnea | 7 (7) | 14 (13) |
|
Musculoskeletal and connective tissue disorders |
||
| Back pain | 13 (12) | 10 (9) |
| Muscular weakness | 6 (6) | 4 (4) |
| Renal and urinary disorders | ||
| Renal failure | 10 (9) | 7 (6) |
| a POMALYST alone arm includes all patients randomized to the POMALYST alone arm who took study drug; 61 of the 107 patients had dexamethasone added during the treatment period. | ||
| Trial 1 | ||
|
POMALYSTa
(n=107) |
POMALYST + Low-dose Dex (n=112) |
|
| System Organ Class/Preferred Term | n (%) | n (%) |
| Patients With at Least One Treatment- Emergent Serious Adverse Reaction |
72 (67) | 69 (62) |
| Infections and infestations | ||
| Pneumonia | 15 (14) | 21 (19) |
| Urinary tract infection | 0 (0) | 6 (5) |
| Sepsis | 6 (6) | 3 (3) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dyspnea | 5 (5) | 7 (6) |
|
General disorders and administration site conditions |
||
| Pyrexia | 3 (3) | 5 (5) |
| General physical health deterioration | 0 (0) | 2 (2) |
| Cardiac disorders | ||
| Atrial fibrillation | 2 (2) | 3 (3) |
| Cardiac failure congestive | 0 (0) | 3 (3) |
| Renal and urinary disorders | ||
| Renal failure | 9 (8) | 7 (6) |
| Gastrointestinal disorders | ||
| Constipation | 1 (1) | 3 (3) |
| Blood and lymphatic system disorders | ||
| Febrile neutropenia | 5 (5) | 1 (1) |
| Metabolism and nutrition disorders | ||
| Dehydration | 5 (5) | 3 (3) |
| Hypercalcemia | 5 (5) | 2 (2) |
|
Musculoskeletal and connective tissue disorders |
||
| Back pain | 4 (4) | 2 (2) |
Other Adverse Reactions
Other adverse reactions of POMALYST in patients with multiple myeloma, not described above, and considered important:
Ear and labyrinth disorders: Vertigo
Hepatobiliary disorders: Hyperbilirubinemia
Infections and infestations: Pneumocystis jiroveci pneumonia, Respiratory syncytial virus infection, Neutropenic sepsis
Investigations: Alanine aminotransferase increased
Metabolism and nutritional disorders: Hyperkalemia
Renal and urinary disorders: Urinary retention
Reproductive system and breast disorders: Pelvic pain
Respiratory, thoracic and mediastinal disorders: Interstitial lung disease
6.2 Postmarketing Experience
The following adverse drug reactions have been identified from the worldwide post-marketing experience with POMALYST: Pancytopenia, tumor lysis syndrome.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
Pomalidomide is primarily metabolized by CYP1A2 and CYP3A. Pomalidomide is also a substrate for P-glycoprotein (P-gp).
7.1 Drugs That May Increase Pomalidomide Plasma Concentrations
CYP1A2 inhibitors: Pomalidomide exposure is increased when POMALYST is co-administered with a strong CYP1A2 inhibitor (fluvoxamine) in the presence of a strong CYP3A4/5 and P-gp inhibitor (ketoconazole). Ketoconazole in the absence of a CYP1A2 inhibitor does not increase pomalidomide exposure. Avoid co-administration of strong CYP1A2 inhibitors (e.g. ciprofloxacin and fluvoxamine) [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. If it is medically necessary to co-administer strong inhibitors of CYP1A2 in the presence of strong inhibitors of CYP3A4 and P-gp, POMALYST dose should be reduced by 50%.
The effect of a CYP1A2 inhibitor in the absence of a co-administered CYP3A4 and P-gp inhibitor has not been studied. Monitor for toxicities if CYP1A2 inhibitors are to be co-administered in the absence of a co-administered CYP3A4 and P-gp inhibitor, and reduce dose if needed.
7.2 Drugs That May Decrease Pomalidomide Plasma Concentrations
Smoking: Cigarette smoking may reduce pomalidomide exposure due to CYP1A2 induction. Patients should be advised that smoking may reduce the efficacy of pomalidomide.
CYP1A2 inducers: Co-administration of POMALYST with drugs that are CYP1A2 inducers has not been studied and may reduce pomalidomide exposure.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X [see Boxed Warnings and Contraindications (4) ]
Risk Summary
POMALYST can cause embryo-fetal harm when administered to a pregnant female and is contraindicated during pregnancy. POMALYST is a thalidomide analogue.
Thalidomide is a human teratogen, inducing a high frequency of severe and life-threatening birth defects such as amelia (absence of limbs), phocomelia (short limbs), hypoplasticity of the bones, absence of bones, external ear abnormalities (including anotia, micropinna, small or absent external auditory canals), facial palsy, eye abnormalities (anophthalmos, microphthalmos), and congenital heart defects. Alimentary tract, urinary tract, and genital malformations have also been documented and mortality at or shortly after birth has been reported in about 40% of infants.
Pomalidomide was teratogenic in both rats and rabbits when administered during the period of organogenesis. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
If pregnancy does occur during treatment, immediately discontinue the drug. Under these conditions, refer patient to an obstetrician/gynecologist experienced in reproductive toxicity for further evaluation and counseling. Report any suspected fetal exposure to POMALYST to the FDA via the MedWatch program at 1-800-FDA-1088 and also to Celgene Corporation at 1-888-423-5436.
Animal Data
Pomalidomide was teratogenic in both rats and rabbits in the embryo-fetal developmental studies when administered during the period of organogenesis.
In rats, pomalidomide was administered orally to pregnant animals at doses of 25 to 1000 mg/kg/day. Malformations or absence of urinary bladder, absence of thyroid gland, and fusion and misalignment of lumbar and thoracic vertebral elements (vertebral, central, and/or neural arches) were observed at all dose levels. There was no maternal toxicity observed in this study. The lowest dose in rats resulted in an exposure (AUC) approximately 85-fold of the human exposure at the recommended dose of 4 mg/day. Other embryo-fetal toxicities included increased resorptions leading to decreased number of viable fetuses.
In rabbits, pomalidomide was administered orally to pregnant animals at doses of 10 to 250 mg/kg/day. Increased cardiac malformations such as interventricular septal defect were seen at all doses with significant increases at 250 mg/kg/day. Additional malformations observed at 250 mg/kg/day included anomalies in limbs (flexed and/or rotated fore- and/or hindlimbs, unattached or absent digit) and associated skeletal malformations (not ossified metacarpal, misaligned phalanx and metacarpal, absent digit, not ossified phalanx, and short not ossified or bent tibia), moderate dilation of the lateral ventricle in the brain, abnormal placement of the right subclavian artery, absent intermediate lobe in the lungs, low-set kidney, altered liver morphology, incompletely or not ossified pelvis, an increased average for supernumerary thoracic ribs, and a reduced average for ossified tarsals. No maternal toxicity was observed at the low dose (10 mg/kg/day) that resulted in cardiac anomalies in fetuses; this dose resulted in an exposure (AUC) approximately equal to that reported in humans at the recommended dose of 4 mg/day. Additional embryo-fetal toxicity included increased resorption.
8.3 Nursing Mothers
It is not known if pomalidomide is excreted in human milk. Pomalidomide was excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from POMALYST, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of POMALYST in patients below the age of 18 years have not been established.
8.5 Geriatric Use
No dosage adjustment is required for POMALYST based on age.
Of the total number of patients in clinical studies of POMALYST, 41% were aged 65 years and older, while 12% were aged 75 years and older. No overall differences in effectiveness were observed between these patients and younger patients. In this study, patients aged greater than or equal to 65 years were more likely to experience pneumonia than patients aged less than or equal to 65 years.
8.6 Females of Reproductive Potential and Males
POMALYST can cause fetal harm when administered during pregnancy [see Use in Specific Populations (8.1)]. Females of reproductive potential must avoid pregnancy while taking POMALYST and for at least 4 weeks after completing therapy.
Females
Females of reproductive potential must commit either to abstain continuously from heterosexual sexual intercourse or to use 2 methods of reliable birth control simultaneously: one highly effective form of contraception – tubal ligation, IUD, hormonal (birth control pills, injections, hormonal patches, vaginal rings, or implants), or partner’s vasectomy, and 1 additional effective contraceptive method – male latex or synthetic condom, diaphragm, or cervical cap. Contraception must begin 4 weeks prior to initiating treatment with POMALYST, during therapy, during dose interruptions, and continuing for 4 weeks following discontinuation of POMALYST therapy. Reliable contraception is indicated even where there has been a history of infertility, unless due to hysterectomy. Females of reproductive potential should be referred to a qualified provider of contraceptive methods, if needed.
Females of reproductive potential must have 2 negative pregnancy tests before initiating POMALYST. The first test should be performed within 10-14 days, and the second test within 24 hours prior to prescribing POMALYST. Once treatment has started and during dose interruptions, pregnancy testing for females of reproductive potential should occur weekly during the first 4 weeks of use, then pregnancy testing should be repeated every 4 weeks in females with regular menstrual cycles. If menstrual cycles are irregular, the pregnancy testing should occur every 2 weeks. Pregnancy testing and counseling should be performed if a patient misses her period or if there is any abnormality in her menstrual bleeding. POMALYST treatment must be discontinued during this evaluation.
Males
Pomalidomide is present in the semen of males who take POMALYST. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 28 days after discontinuing POMALYST, even if they have undergone a successful vasectomy. Male patients taking POMALYST must not donate sperm.
8.7 Renal Impairment
Pomalidomide and its metabolites are primarily excreted by the kidneys [see Clinical Pharmacology (12.3)]. The influence of renal impairment on the safety, efficacy, and pharmacokinetics of pomalidomide has not been evaluated. Patients with serum creatinine greater than 3.0 mg/dL were excluded in clinical studies. Avoid POMALYST in patients with a serum creatinine greater than 3.0 mg/dL.
8.8 Hepatic Impairment
Pomalidomide is metabolized in the liver [see Clinical Pharmacology (12.3)]. The influence of hepatic impairment on the safety, efficacy, and pharmacokinetics of pomalidomide has not been evaluated. Patients with serum bilirubin greater than 2.0 mg/dL and AST/ALT greater than 3.0 x upper limit normal (ULN) were excluded in clinical studies. Avoid POMALYST in patients with serum bilirubin greater than 2.0 mg/dL and AST/ALT greater than 3.0 x ULN.
10 OVERDOSAGE
No specific information is available on the treatment of overdose with pomalidomide, and it is unknown whether pomalidomide or its metabolites are dialyzable.
11 DESCRIPTION
POMALYST is an immunomodulatory antineoplastic agent. The chemical name is (RS)-4-Amino-2-(2,6-dioxo-piperidin-3-yl)-isoindoline-1,3-dione and it has the following chemical structure:
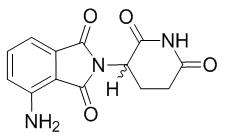
The empirical formula for pomalidomide is C13H11N3O4 and the gram molecular weight is 273.24.
Pomalidomide is a yellow solid powder. It has limited to low solubility into organic solvents and it has low solubility in all pH solutions (about 0.01 mg/mL). Pomalidomide has a chiral carbon atom which exists as a racemic mixture of the R(+) and S(-) enantiomers.
POMALYST is available in 1-mg, 2-mg, 3-mg, and 4-mg capsules for oral administration. Each capsule contains pomalidomide as the active ingredient and the following inactive ingredients: mannitol, pregelatinized starch, and sodium stearyl fumarate. The 1-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, white ink, and black ink. The 2-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, FD&C red 3, and white ink. The 3-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, and white ink. The 4-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 1, FD&C blue 2, and white ink.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pomalidomide, an analogue of thalidomide, is an immunomodulatory agent with antineoplastic activity. In in vitro cellular assays, pomalidomide inhibited proliferation and induced apoptosis of hematopoietic tumor cells. Additionally, pomalidomide inhibited the proliferation of lenalidomide-resistant multiple myeloma cell lines and synergized with dexamethasone in both lenalidomide-sensitive and lenalidomide-resistant cell lines to induce tumor cell apoptosis. Pomalidomide enhanced T cell- and natural killer (NK) cell-mediated immunity and inhibited production of pro-inflammatory cytokines (eg, TNF-α and IL-6) by monocytes. Pomalidomide demonstrated anti-angiogenic activity in a mouse tumor model and in the in vitro umbilical cord model.
12.3 Pharmacokinetics
Absorption
Following administration of single oral doses of POMALYST, the maximum plasma concentration (Cmax) for pomalidomide occurs at 2 and 3 hours postdose. The systemic exposure (AUC) of pomalidomide increases in an approximately dose proportional manner.
In patients with multiple myeloma who received POMALYST 4 mg daily alone or in combination with dexamethasone, pomalidomide steady-state drug exposure was characterized by AUC(Τ) of 400 ng•h/mL and Cmax of 75 ng/mL. Following multiple doses, pomalidomide has an accumulation ratio of 27% to 31%.
Distribution
Pomalidomide has a mean apparent volume of distribution (Vd/F) between 62 and 138 L at steady state. Pomalidomide is distributed in semen of healthy subjects at a concentration of approximately 67% of plasma level at 4 hours postdose (~Tmax) after 4 days of once-daily dosing at 2 mg. Human plasma protein binding ranges from 12% to 44% and is not concentration dependent. Pomalidomide is a substrate for P-glycoprotein (P-gp).
Metabolism
Pomalidomide is primarily metabolized in the liver by CYP1A2 and CYP3A4. In vitro, CYP1A2 and CYP3A4 were identified as the primary enzymes involved in the CYP-mediated hydroxylation of pomalidomide, with additional minor contributions from CYP2C19 and CYP2D6.
Elimination
Pomalidomide is eliminated with a median plasma half-life of approximately 9.5 hours in healthy subjects and approximately 7.5 hours in patients with multiple myeloma. Pomalidomide has a mean total body clearance (CL/F) of 7-10 L/h.
Following a single oral administration of [14C]-pomalidomide (2 mg) to healthy subjects, approximately 73% and 15% of the radioactive dose was eliminated in urine and feces, respectively, with approximately 2% and 8% of the radiolabeled dose eliminated unchanged as pomalidomide in urine and feces.
Drug Interactions
Drugs that Inhibit Pomalidomide Metabolism
CYP1A2 Inhibitors: The effect of CYP1A2 inhibitors, in the absence of a co-administered CYP3A4 and P-gp inhibitor, is unknown. However, co-administration of fluvoxamine (a strong CYP1A2 inhibitor) in the presence of ketoconazole (a strong CYP3A4 and P-gp inhibitor) to 12 healthy male subjects increased exposure (geometric mean AUCINF) to pomalidomide by 146% compared to pomalidomide administered alone [see Dosage and Administration (2.2) and Drug Interactions (7.1)].
Strong CYP3A4 and P-glycoprotein (P-gp) Inhibitors: Co-administration of ketoconazole (a strong CYP3A4 and P-gp inhibitor) in 16 healthy male subjects resulted in an increased exposure (geometric mean AUCINF) to pomalidomide of 19% compared to pomalidomide administered alone.
Drugs that Induce Pomalidomide Metabolism
Strong CYP1A2 Inducers: Co-administration of POMALYST with drugs that are CYP1A2 inducers has not been studied and may reduce pomalidomide exposure.
Strong CYP3A4 Inducers: Co-administration of carbamazepine to 16 healthy male subjects decreased exposure (geometric mean AUCINF) to pomalidomide by 21% compared to pomalidomide administered alone.
Dexamethasone: Co-administration of multiple doses of 4 mg POMALYST with 20 mg to 40 mg dexamethasone (a weak to moderate inducer of CYP3A4) to patients with multiple myeloma had no effect on the pharmacokinetics of pomalidomide compared with pomalidomide administered alone.
In Vitro Inhibition of Drug Metabolizing Enzymes and Transporters by Pomalidomide
Pomalidomide does not inhibit or induce CYP450 enzymes or transporters in vitro.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies examining the carcinogenic potential of pomalidomide have not been conducted. One of 12 monkeys dosed with 1 mg/kg of pomalidomide (an exposure approximately 15-fold of the exposure in patients at the recommended dose of 4 mg/day) developed acute myeloid leukemia in a 9-month repeat-dose toxicology study.
Pomalidomide was not mutagenic or clastogenic in a battery of tests, including the bacteria reverse mutation assay (Ames test), the in vitro assay using human peripheral blood lymphocytes, and the micronucleus test in orally treated rats administered doses up to 2000 mg/kg/day.
In a fertility and early embryonic development study in rats, drug-treated males were mated with untreated or treated females. Pomalidomide was administered to males and females at doses of 25 to 1000 mg/kg/day. When treated males were mated with treated females, there was an increase in post-implantation loss and a decrease in mean number of viable embryos at all dose levels. There were no other effects on reproductive functions or the number of pregnancies. The lowest dose tested in animals resulted in an exposure (AUC) approximately 100-fold of the exposure in patients at the recommended dose of 4 mg/day. When treated males in this study were mated with untreated females, all uterine parameters were comparable to the controls. Based on these results, the observed effects were attributed to the treatment of females.
14 CLINICAL STUDIES
14.1 Multiple Myeloma
Trial 1 was a phase 2, multicenter, randomized open-label study in patients with relapsed multiple myeloma who were refractory to their last myeloma therapy and had received lenalidomide and bortezomib. Patients were considered relapsed if they had achieved at least stable disease for at least 1 cycle of treatment to at least 1 prior regimen and then developed progressive disease. Patients were considered refractory if they experienced disease progression on or within 60 days of their last therapy. A total of 221 patients were randomized to receive POMALYST alone or POMALYST with Low-dose Dex. In Trial 1, the safety and efficacy of POMALYST 4 mg, once daily for 21 of 28 days, until disease progression, were evaluated alone and in combination with Low-dose Dex (40 mg/day given only on Days 1, 8, 15, and 22 of each 28-day cycle for patients aged 75 years or younger, or 20 mg/day given only on Days 1, 8, 15, and 22 of each 28-day cycle for patients aged greater than 75 years). Patients in the POMALYST alone arm were allowed to add Low-dose Dex upon disease progression.
Table 5 summarizes the baseline patient and disease characteristics in Trial 1. The baseline demographics and disease characteristics were balanced and comparable between the study arms.
|
POMALYST (n=108) |
POMALYST + Low-dose Dex (n=113) |
|
| Patient Characteristics | ||
| Median age, years (range) |
61 (37-88) | 64 (34-88) |
| Age distribution, n (%) <65 years ≥65 years |
65 (60.2) 43 (39.8) |
60 (53.1) 53 (46.9) |
| Sex, n (%) Male Female |
57 (52.8) 51 (47.2) |
62 (54.9) 51 (45.1) |
| Race/ethnicity, n (%) White Black or African American All other race |
86 (79.6) 16 (14.8) 6 (5.6) |
92 (81.4) 17 (15) 4 (3.6) |
| ECOG Performance, n (%) Status 0-1 |
95 (87.9) | 100 (88.5) |
| Disease Characteristics | ||
| Number of prior therapies Median, (min, max) |
5 (2, 12) | 5 (2, 13) |
| Prior transplant, n (%) | 82 (75.9) | 84 (74.3) |
| Refractory to bortezomib and lenalidomide, n (%) |
64 (59.3) | 69 (61.1) |
Table 6 summarizes the analysis results of overall response rate (ORR) and duration of response (DOR), based on assessments by the Independent Review Adjudication Committee for the treatment arms in Study 1. ORR did not differ based on type of prior anti-myeloma therapy.
|
a Results are prior to the addition of dexamethasone.
b ORR = PR + CR per EBMT criteria. CI, confidence interval; NE, not established (the median has not yet been reached). |
||
|
POMALYST a
(n=108) |
POMALYST + Low-dose Dex (n=113) |
|
| Response | ||
| Overall Response Rate (ORR),b n (%) |
8 (7.4) | 33 (29.2) |
| 95% CI for ORR (%) | (3.3, 14.1) | (21.0, 38.5) |
| Complete Response (CR), n (%) | 0 (0.0) | 1 (0.9) |
| Partial Response (PR), n (%) | 8 (7.4) | 32 (28.3) |
| Duration of Response (DOR) | ||
| Median, months | NE | 7.4 |
| 95% CI for DOR (months) | NE | (5.1, 9.2) |
15 REFERENCES
1. OSHA Hazardous Drugs. OSHA. [Accessed on 29 January 2013, from http://www.osha.gov/SLTC/hazardousdrugs/index.html]
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Dark blue opaque cap and yellow opaque body, imprinted “POML” on the cap in white ink and “1 mg” on the body in black ink
1 mg bottles of 21 (NDC 59572-501-21)
1 mg bottles of 100 (NDC 59572-501-00)
Dark blue opaque cap and orange opaque body, imprinted “POML” on the cap and “2 mg” on the body in white ink
2 mg bottles of 21 (NDC 59572-502-21)
2 mg bottles of 100 (NDC 59572-502-00)
Dark blue opaque cap and green opaque body, imprinted “POML” on the cap and “3 mg” on the body in white ink
3 mg bottles of 21 (NDC 59572-503-21)
3 mg bottles of 100 (NDC 59572-503-00)
Dark blue opaque cap and blue opaque body, imprinted “POML” on the cap and “4 mg” on the body in white ink
4 mg bottles of 21 (NDC 59572-504-21)
4 mg bottles of 100 (NDC 59572-504-00)
16.2 Storage
Store at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F-86°F). [See USP Controlled Room Temperature].
16.3 Handling and Disposal
Care should be exercised in handling of POMALYST. POMALYST capsules should not be opened or crushed. If powder from POMALYST contacts the skin, wash the skin immediately and thoroughly with soap and water. If POMALYST contacts the mucous membranes, flush thoroughly with water.
Follow procedures for proper handling and disposal of anticancer drugs.1
17 PATIENT COUNSELING INFORMATION
See FDA-approved Patient Labeling ( Medication Guide )
Embryo-Fetal Toxicity
Advise patients that POMALYST is contraindicated in pregnancy [see Contraindications (4)]. POMALYST is a thalidomide analogue and may cause serious birth defects or death to a developing baby [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- Advise females of reproductive potential that they must avoid pregnancy while taking POMALYST and for at least 4 weeks after completing therapy.
- Initiate POMALYST treatment in females of reproductive potential only following a negative pregnancy test.
- Advise females of reproductive potential of the importance of monthly pregnancy tests and the need to use 2 different forms of contraception, including at least 1 highly effective form, simultaneously during POMALYST therapy, during therapy interruption, and for 4 weeks after she has completely finished taking POMALYST. Highly effective forms of contraception other than tubal ligation include IUD and hormonal (birth control pills, injections, patch, or implants) and a partner’s vasectomy. Additional effective contraceptive methods include latex or synthetic condom, diaphragm, and cervical cap.
- Instruct patient to immediately stop taking POMALYST and contact her doctor if she becomes pregnant while taking this drug, if she misses her menstrual period or experiences unusual menstrual bleeding, if she stops taking birth control, or if she thinks FOR ANY REASON that she may be pregnant.
- Advise patient that if her doctor is not available, she can call 1-888-668-2528 for information on emergency contraception [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
- Advise males to always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 28 days after discontinuing POMALYST, even if they have undergone a successful vasectomy.
- Advise male patients taking POMALYST that they must not donate sperm [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
- All patients must be instructed to not donate blood while taking POMALYST and for 1 month following discontinuation of POMALYST [see Warnings and Precautions (5.1) and Use in Specific Populations (8.6)].
POMALYST REMS Program
Because of the risk of embryo-fetal toxicity, POMALYST is only available through a restricted program called POMALYST REMS [see Warnings and Precautions (5.2)].
- Patients must sign a Patient-Prescriber agreement form and comply with the requirements to receive POMALYST. In particular, females of reproductive potential must comply with the pregnancy testing, contraception requirements and participate in monthly telephone surveys. Males must comply with the contraception requirements [see Use in Specific Populations (8.6)].
- POMALYST is available only from pharmacies that are certified in POMALYST REMS. Provide patients with the telephone number and Web site for information on how to obtain the product.
Venous Thromboembolism
Inform patients of the potential risk of developing venous thromboembolic events and discuss the need for appropriate prophylactic treatment [see Venous Thromboembolism (5.3)].
Hematologic Toxicities
Inform patients on the risks of developing neutropenia, thrombocytopenia, and anemia and the need to report signs and symptoms associated with these events to their healthcare provider for further evaluation [see Hematologic Toxicities (5.4)].
Hypersensitivity
Inform patients of the potential for a severe hypersensitivity reaction to POMALYST if they have had such a reaction in the past to either THALOMID® or REVLIMID® [see Hypersensitivity Reaction (5.5)].
Dizziness and Confusional State
Inform patients of the potential risk of dizziness and confusional state with the drug, to avoid situations where dizziness or confusional state may be a problem, and not to take other medications that may cause dizziness or confusional state without adequate medical advice [see Dizziness and Confusional State (5.6)].
Neuropathy
Inform patients of the risk of neuropathy and to report the signs and symptoms associated with these events to their healthcare provider for further evaluation [see Neuropathy (5.7)].
Second Primary Malignancies
Inform the patient that the potential risk of developing acute myelogenous leukemia during treatment with POMALYST is unknown [see Risk of Second Primary Malignancies (5.8)].
Tumor Lysis Syndrome
Inform patients of the potential risk of tumor lysis syndrome and to report any signs and symptoms associated with this event to their healthcare provider for evaluation [see Warning and Precautions (5.9)].
Dosing Instructions
Inform patients on how to take POMALYST [see Dosage and Administration (2.1)]
- POMALYST should be taken once daily at about the same time each day.
- POMALYST should be taken without food (at least 2 hours before or 2 hours after a meal).
- The capsules should not be opened, broken, or chewed. POMALYST should be swallowed whole with water.
- Instruct patients that if they miss a dose of POMALYST, they may still take it up to 12 hours after the time they would normally take it. If more than 12 hours have elapsed, they should be instructed to skip the dose for that day. The next day, they should take POMALYST at the usual time. Warn patients not to take 2 doses to make up for the one that they missed.
Other Information
Advise patients who smoke to stop because smoking may reduce the efficacy of pomalidomide [see Drug Interactions (7.2)].
Manufactured for: Celgene Corporation
Summit, NJ 07901
POMALYST®, REVLIMID®, and THALOMID® are registered trademarks of Celgene
Corporation.
POMALYST REMS™ is a trademark of Celgene Corporation.
Pat. http://www.celgene.com/therapies
© 2005-2014 Celgene Corporation All rights reserved.
POMPI.003/MG.003 05/2014
MEDICATION GUIDE
POMALYST® (POM-uh-list)
(pomalidomide)
capsules
Read the Medication Guide that comes with POMALYST before you start taking it and each time you get a new prescription. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about POMALYST?
- Before you begin taking POMALYST, you must read and agree to all of the instructions in the POMALYST REMS™ program.
- POMALYST may cause serious side effects including:
Possible birth defects (deformed babies) or death of an unborn baby. Females who are pregnant or who plan to become pregnant must not take POMALYST.
POMALYST is similar to the medicine thalidomide (THALOMID). We know thalidomide can cause severe life-threatening birth defects. POMALYST has not been tested in pregnant women. POMALYST has harmed unborn animals in animal testing.
Females must not get pregnant:- for at least 4 weeks before starting POMALYST
- while taking POMALYST
- during any breaks (interruptions) in your treatment with POMALYST
- for at least 4 weeks after stopping POMALYST
If you become pregnant while taking POMALYST, stop taking it right away and call your healthcare provider. If your healthcare provider is not available, you can call 1-888-668-2528 for medical information. Healthcare providers and patients should report all cases of pregnancy to:- FDA MedWatch at 1-800-FDA-1088, and
- Celgene Corporation at 1-888-423-5436
POMALYST can pass into human semen:- Males, including those who have had a vasectomy, must use a latex or synthetic condom during any sexual contact with a pregnant female or a female that can become pregnant while taking POMALYST, during any breaks (interruptions) in your treatment with POMALYST, and for 4 weeks after stopping POMALYST.
- Do not have unprotected sexual contact with a female who is or could become pregnant. Tell your healthcare provider if you do have unprotected sexual contact with a female who is or could become pregnant.
- Do not donate sperm while taking POMALYST, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping POMALYST. If a female becomes pregnant with your sperm, the baby may be exposed to POMALYST and may be born with birth defects.
Men, if your female partner becomes pregnant, you should call your healthcare provider right away.
-
Blood clots in your veins and lungs. If you take POMALYST, you may have an increased risk for blood clots in your veins and lungs. Call your healthcare provider or get medical help right away if you get any of these signs or symptoms while taking POMALYST:
- shortness of breath
- chest pain
- arm or leg swelling
What is POMALYST?
POMALYST is a prescription medicine used to treat people with multiple myeloma who:
- have received at least 2 prior medicines to treat multiple myeloma, including bortezomib and lenalidomide, and
- their disease has become worse during treatment or within 60 days of finishing the last treatment.
It is not known if POMALYST is safe and effective in people under 18 years of age.
Who should not take POMALYST?
Do not take POMALYST if you are pregnant, plan to become pregnant, or become pregnant during treatment with POMALYST. See “What is the most important information I should know about POMALYST?”
What should I tell my healthcare provider before taking POMALYST?
Before you take POMALYST, tell your healthcare provider if you:
- have a history of serious allergic reactions to thalidomide (THALOMID) or lenalidomide (REVLIMID). You may be at risk if you take POMALYST if you have had serious allergic reactions to these medicines
- smoke cigarettes
- have any other medical condition
- are breast-feeding. POMALYST must not be used by women who are breast-feeding. It is not known if POMALYST passes into your breast milk and can harm your baby
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. POMALYST and other medicines may affect each other causing serious side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist.
How should I take POMALYST?
Take POMALYST exactly as prescribed and follow all the instructions of the POMALYST REMS program.
Before prescribing POMALYST, your healthcare provider will:
-
- explain the POMALYST REMS program to you
- have you sign the Patient-Physician Agreement Form
- Swallow POMALYST capsules whole with water 1 time a day. Do not break, chew, or open your capsules.
- Take POMALYST at about the same time each day.
-
POMALYST should be taken without food, at least 2 hours before or 2 hours after a meal.
- Do not open the POMALYST capsules or handle them any more than needed. If you touch a broken POMALYST capsule or the medicine in the capsule, wash the area of your body right away with soap and water.
- If you miss a dose of POMALYST and it has been less than 12 hours since your regular time, take it as soon as you remember. If it has been more than 12 hours, just skip your missed dose. Do not take 2 doses at the same time.
- If you take too much POMALYST, call your healthcare provider right away.
Females who can become pregnant:
- will have pregnancy tests weekly for 4 weeks, then every 4 weeks if your menstrual cycle is regular, or every 2 weeks if your menstrual cycle is irregular.
If you miss your period or have unusual bleeding, you will need to have a pregnancy test and receive counseling.
- must agree to use 2 different forms of effective birth control at the same time, for at least 4 weeks before, while taking, during any breaks (interruptions) in your treatment, and for at least 4 weeks after stopping POMALYST.
Males who take POMALYST, even those who have had a vasectomy, must agree to use a latex or synthetic condom during sexual contact with a pregnant female or a female who can become pregnant.
What should I avoid while taking POMALYST?
- See “What is the most important information I should know about POMALYST?”
-
Females: Do not get pregnant and do not breast-feed while taking POMALYST.
Males: Do not donate sperm.
-
Do not share POMALYST with other people. It may cause birth defects and other serious problems.
-
Do not donate blood while you take POMALYST, during any breaks (interruptions) in your treatment, and for 4 weeks after stopping POMALYST. If someone who is pregnant gets your donated blood, her baby may be exposed to POMALYST and may be born with birth defects.
- You should not smoke cigarettes while taking POMALYST. Smoking cigarettes during treatment with POMALYST may affect how well POMALYST works.
What are the possible side effects of POMALYST?
POMALYST may cause serious side effects, including:
- See “What is the most important information I should know about POMALYST?”
-
Low white blood cells (neutropenia), low platelets (thrombocytopenia), and low red blood cells (anemia). POMALYST may cause low white blood cells, low platelets, and low red blood cells. You may need a blood transfusion or certain medicines if your blood counts drop too low. Your blood counts should be checked weekly for the first 8 weeks of treatment and monthly thereafter.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
The most common side effects of POMALYST include:
- tiredness and weakness
- constipation
- shortness of breath
- diarrhea
- fever
- back pain
- nausea
These are not all the possible side effects of POMALYST.
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store POMALYST?
- Store POMALYST at room temperature (68°F to 77°F [20°C to 25°C]) with excursions permitted to 59°F to 86°F (15°C to 30°C).
- Return any unused POMALYST to Celgene or your healthcare provider.
Keep POMALYST and all medicines out of the reach of children.
General information about POMALYST
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take POMALYST for conditions for which it was not prescribed. Do not give POMALYST to other people, even if they have the same symptoms you have. It may harm them and may cause birth defects.
This Medication Guide provides a summary of the most important information about POMALYST. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about POMALYST that is written for healthcare providers.
For more information, call 1-888-423-5436 or go to www.CelgeneRiskManagement.com.
What are the ingredients in POMALYST?
Active ingredient: pomalidomide
Inactive ingredients: mannitol, pregelatinized starch, and sodium stearyl fumarate.
The 1-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, white ink, and black ink.
The 2-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, FD&C red 3, and white ink.
The 3-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, and white ink.
The 4-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 1, FD&C blue 2, and white ink.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued 05/2014
Manufactured for:
Celgene Corporation
Summit, NJ 07901
POMALYST®, REVLIMID®, and THALOMID® are registered trademarks of Celgene Corporation.
POMALYST REMS™ is a trademark of Celgene Corporation.
Pat. http://www.celgene.com/therapies
© 2005-2014 Celgene Corporation All rights reserved.
POMMG.003 05/2014
Pomalyst® (pomalidomide) Capsules, 1 mg - 21 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 1 mg - 100 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 2 mg - 21 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 2 mg - 100 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 3 mg - 21 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 3 mg - 100 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 4 mg - 21 Count Bottle Label

Pomalyst® (pomalidomide) Capsules, 4 mg - 100 Count Bottle Label

Pomalystpomalidomide CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pomalystpomalidomide CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pomalystpomalidomide CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pomalystpomalidomide CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||