Pradaxa
Boehringer Ingelheim Pharmaceuticals Inc.
Boehringer Ingelheim Pharmaceuticals Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRADAXA safely and effectively. See full prescribing information for PRADAXA. PRADAXA® (dabigatran etexilate mesylate) capsules for oral useInitial U.S. Approval: 2010BOXED WARNING WARNING: (A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS, and (B) SPINAL/EPIDURAL HEMATOMA See full prescribing information for complete boxed warning (A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS: Premature discontinuation of any oral anticoagulant, including PRADAXA, increases the risk of thrombotic events. To reduce this risk, consider coverage with another anticoagulant if PRADAXA is discontinued for a reason other than pathological bleeding or completion of a course of therapy (2.4, 2.5, 2.6, 5.1). (B) SPINAL/EPIDURAL HEMATOMA: Epidural or spinal hematomas may occur in patients treated with PRADAXA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis (5.3). Monitor patients frequently for signs and symptoms of neurological impairment and if observed, treat urgently. Consider the benefits and risks before neuraxial intervention in patients who are or who need to be anticoagulated (5.3). RECENT MAJOR CHANGES Boxed Warning 4/2014 Indications and Usage (1.1, 1.2, 1.3) 4/2014 Dosage and Administration (2.1, 2.2) 4/2014 Dosage and Administration (2.3) 12/2013 Warnings and Precautions (5.1, 5.3, 5.5) 4/2014 INDICATIONS AND USAGEPRADAXA is a direct thrombin inhibitor indicated: To reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation (1.1) For the treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE) in patients who have been treated with a parenteral anticoagulant for 5-10 days (1.2) To reduce the risk of recurrence of DVT and PE in patients who have been previously treated (1.3) DOSAGE AND ADMINISTRATION Non-valvular Atrial Fibrillation: For patients with CrCl >30 mL/min: 150 mg orally, twice daily (2.1) For patients with CrCl 15-30 mL/min: 75 mg orally, twice daily (2.1) Treatment and Reduction in the Risk of Recurrence of DVT and PE: For patients with CrCl >30 mL/min: 150 mg orally, twice daily after 5-10 days of parenteral anticoagulation (2.1) Instruct patients not to chew, break, or open capsules (2.3) Review recommendations for converting to or from other oral or parenteral anticoagulants (2.4, 2.5) Temporarily discontinue PRADAXA before invasive or surgical procedures when possible, then restart promptly (2.6) DOSAGE FORMS AND STRENGTHSCapsules: 75 mg and 150 mg (3) CONTRAINDICATIONS Active pathological bleeding (4) History of serious hypersensitivity reaction to PRADAXA (4) Mechanical prosthetic heart valve (4) WARNINGS AND PRECAUTIONS Bleeding: PRADAXA can cause serious and fatal bleeding (5.2) Bioprosthetic heart valves: PRADAXA use not recommended (5.4) Side EffectsMost common adverse reactions (>15%) are gastritis-like symptoms and bleeding (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or (800) 459-9906 TTY or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .DRUG INTERACTIONS P-gp inducers rifampin: Avoid coadministration with PRADAXA (5.5) P-gp inhibitors in patients with CrCl 30-50 mL/min: Consider reducing dose or avoid (7) P-gp inhibitors in patients with CrCl
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: (A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA
- 1 PRADAXA INDICATIONS AND USAGE
- 2 PRADAXA DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 PRADAXA CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 PRADAXA ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 PRADAXA DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
WARNING: (A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA
(A)
PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC
EVENTS
Premature discontinuation of any oral anticoagulant,
including PRADAXA, increases the risk of thrombotic events. If anticoagulation
with PRADAXA is discontinued for a reason other than pathological
bleeding or completion of a course of therapy, consider coverage with
another anticoagulant [see Dosage and Administration (2.4, 2.5, 2.6) and Warnings and Precautions (5.1)].
(B) SPINAL/EPIDURAL HEMATOMA
Epidural or spinal
hematomas may occur in patients treated with PRADAXA who are receiving
neuraxial anesthesia or undergoing spinal puncture. These hematomas
may result in long-term or permanent paralysis. Consider these risks
when scheduling patients for spinal procedures. Factors that can increase
the risk of developing epidural or spinal hematomas in these patients
include:
• use of indwelling
epidural catheters
• concomitant use of other drugs that
affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs),
platelet inhibitors, other anticoagulants
• a history
of traumatic or repeated epidural or spinal punctures
•
a history of spinal deformity or spinal surgery
•
optimal timing between the administration of PRADAXA and neuraxial
procedures is not known [see Warnings and Precautions (5.3)].
Monitor patients frequently for signs and symptoms of neurological
impairment. If neurological compromise is noted, urgent treatment
is necessary [see Warnings and Precautions (5.3)].
Consider the benefits and risks before neuraxial intervention in
patients anticoagulated or to be anticoagulated [see Warnings
and Precautions (5.3)].
1 INDICATIONS AND USAGE
1.1 Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
PRADAXA is indicated to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
1.2 Treatment of Deep Venous Thrombosis and Pulmonary Embolism
PRADAXA is indicated for the treatment of deep venous thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5-10 days.
1.3 Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
PRADAXA is indicated to reduce the risk of recurrence of deep venous thrombosis and pulmonary embolism in patients who have been previously treated.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
| Indication | Dosage | |
|---|---|---|
| Reduction in Risk of Stroke and Systemic Embolism in Non-valvular AF |
CrCl >30 mL/min:
|
150 mg twice daily
|
|
CrCl 15 to 30 mL/min:
|
75 mg twice daily
|
|
|
CrCl <15 mL/min or on
dialysis:
|
Dosing recommendations
cannot be provided
|
|
|
CrCl 30 to 50 mL/min with concomitant
use of P-gp inhibitors:
|
Consider reducing dose
to 75 mg twice daily if given with P-gp inhibitors dronedarone or
ketoconazole. Dose adjustment is not necessary when co-administered
with other P-gp inhibitors
|
|
|
CrCl <30 mL/min with
concomitant use of P-gp inhibitors:
|
Avoid co-administration
|
|
|
Treatment of DVT and PE Reduction in the Risk of Recurrence of DVT and PE |
CrCl >30 mL/min:
|
150 mg twice daily
|
|
CrCl <30 mL/min or on
dialysis:
|
Dosing recommendations
cannot be provided
|
|
|
CrCl <50 mL/min with
concomitant use of P-gp inhibitors:
|
Avoid co-administration
|
|
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
For patients with creatinine clearance (CrCl) >30 mL/min, the recommended dose of PRADAXA is 150 mg taken orally, twice daily. For patients with severe renal impairment (CrCl 15-30 mL/min), the recommended dose of PRADAXA is 75 mg twice daily [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with a CrCl <15 mL/min or on dialysis cannot be provided.
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
For patients with CrCl >30 mL/min, the recommended dose of PRADAXA is 150 mg taken orally, twice daily, after 5-10 days of parenteral anticoagulation. Dosing recommendations for patients with a CrCl <30 mL/min or on dialysis cannot be provided [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.2 Dosing Adjustments
Assess renal function prior to initiation of treatment with PRADAXA. Periodically assess renal function as clinically indicated (i.e., more frequently in clinical situations that may be associated with a decline in renal function) and adjust therapy accordingly. Discontinue PRADAXA in patients who develop acute renal failure while on PRADAXA and consider alternative anticoagulant therapy.
Generally, the extent of anticoagulation does not need to be assessed. When necessary, use aPTT or ECT, and not INR, to assess for anticoagulant activity in patients on PRADAXA [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)].
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
In patients with moderate renal impairment (CrCl 30-50 mL/min), concomitant use of the P-gp inhibitor dronedarone or systemic ketoconazole can be expected to produce dabigatran exposure similar to that observed in severe renal impairment. Consider reducing the dose of PRADAXA to 75 mg twice daily [see Warnings and Precautions (5.5), Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
Dosing recommendations for patients with CrCl <30 mL/min cannot be provided. Avoid use of concomitant P-gp inhibitors in patients with CrCl <50 mL/min [see Warnings and Precautions (5.5), Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
2.3 Instructions to Patients
Instruct patients to swallow the capsules whole. PRADAXA should be taken with a full glass of water. Breaking, chewing, or emptying the contents of the capsule can result in increased exposure [see Clinical Pharmacology (12.3)] .
If a dose of PRADAXA is not taken at the scheduled time, the dose should be taken as soon as possible on the same day; the missed dose should be skipped if it cannot be taken at least 6 hours before the next scheduled dose. The dose of PRADAXA should not be doubled to make up for a missed dose.
2.4 Converting from or to Warfarin
When converting patients from warfarin therapy to PRADAXA, discontinue warfarin and start PRADAXA when the INR is below 2.0.
When converting from PRADAXA to warfarin, adjust the starting time of warfarin based on creatinine clearance as follows:
- For CrCl ≥50 mL/min, start warfarin 3 days before discontinuing PRADAXA.
- For CrCl 30-50 mL/min, start warfarin 2 days before discontinuing PRADAXA.
- For CrCl 15-30 mL/min, start warfarin 1 day before discontinuing PRADAXA.
- For CrCl <15 mL/min, no recommendations can be made.
Because PRADAXA can increase INR, the INR will better reflect warfarin’s effect only after PRADAXA has been stopped for at least 2 days [see Clinical Pharmacology (12.2)] .
2.5 Converting from or to Parenteral Anticoagulants
For patients currently receiving a parenteral anticoagulant, start PRADAXA 0 to 2 hours before the time that the next dose of the parenteral drug was to have been administered or at the time of discontinuation of a continuously administered parenteral drug (e.g., intravenous unfractionated heparin).
For patients currently taking PRADAXA, wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of PRADAXA before initiating treatment with a parenteral anticoagulant [see Clinical Pharmacology (12.3)] .
2.6 Discontinuation for Surgery and Other Interventions
If possible, discontinue PRADAXA 1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)] .
If surgery cannot be delayed, there is an increased risk of bleeding [see Warnings and Precautions (5.2)] . This risk of bleeding should be weighed against the urgency of intervention [see Warnings and Precautions (5.1, 5.3)] .
3 DOSAGE FORMS AND STRENGTHS
150 mg capsules with a light blue opaque cap imprinted in black with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted in black with "R150".
75 mg capsules with a cream-colored opaque cap imprinted in black with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted in black with "R75".
4 CONTRAINDICATIONS
PRADAXA is contraindicated in patients with:
- Active pathological bleeding [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)] .
- History of a serious hypersensitivity reaction to PRADAXA (e.g., anaphylactic reaction or anaphylactic shock) [see Adverse Reactions (6.1)] .
- Mechanical prosthetic heart valve [see Warnings and Precautions (5.4)]
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Thrombotic Events after Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including PRADAXA, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. If PRADAXA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant [see Dosage and Administration (2.4, 2.5, 2.6)].
5.2 Risk of Bleeding
PRADAXA increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue PRADAXA in patients with active pathological bleeding [see Dosage and Administration (2.2)] .
Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). PRADAXA’s anticoagulant activity and half-life are increased in patients with renal impairment [see Clinical Pharmacology (12.2)] .
Reversal of Anticoagulant Effect:
A specific reversal agent for dabigatran is not available.
Hemodialysis can remove dabigatran; however the clinical experience
supporting the use of hemodialysis as a treatment for bleeding is
limited [see Overdosage (10)]. Activated prothrombin complex concentrates (aPCCs, e.g.,
FEIBA), or recombinant Factor VIIa, or concentrates of coagulation
factors II, IX or X may be considered but their use has not been evaluated
in clinical trials. Protamine sulfate and vitamin K are not expected
to affect the anticoagulant activity of dabigatran. Consider administration
of platelet concentrates in cases where thrombocytopenia is present
or long-acting antiplatelet drugs have been used.
5.3 Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis [see Boxed Warning].
To reduce the potential risk of bleeding associated with the concurrent use of dabigatran and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dabigatran [see Clinical Pharmacology (12.3)]. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of dabigatran is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
5.4 Thromboembolic and Bleeding Events in Patients with Prosthetic Heart Valves
The safety and efficacy of PRADAXA in patients with bileaflet mechanical prosthetic heart valves was evaluated in the RE-ALIGN trial, in which patients with bileaflet mechanical prosthetic heart valves (recently implanted or implanted more than three months prior to enrollment) were randomized to dose adjusted warfarin or 150, 220, or 300 mg of PRADAXA twice a day. RE-ALIGN was terminated early due to the occurrence of significantly more thromboembolic events (valve thrombosis, stroke, transient ischemic attack, and myocardial infarction) and an excess of major bleeding (predominantly post-operative pericardial effusions requiring intervention for hemodynamic compromise) in the PRADAXA treatment arm as compared to the warfarin treatment arm. These bleeding and thromboembolic events were seen both in patients who were initiated on PRADAXA post-operatively within three days of mechanical bileaflet valve implantation, as well as in patients whose valves had been implanted more than three months prior to enrollment. Therefore, the use of PRADAXA is contraindicated in patients with mechanical prosthetic valves [see Contraindications (4)].
The use of PRADAXA for the prophylaxis of thromboembolic events in patients with atrial fibrillation in the setting of other forms of valvular heart disease, including the presence of a bioprosthetic heart valve, has not been studied and is not recommended.
5.5 Effect of P-gp Inducers and Inhibitors on Dabigatran Exposure
The concomitant use of PRADAXA with P-gp inducers (e.g., rifampin) reduces exposure to dabigatran and should generally be avoided [see Clinical Pharmacology (12.3)].
P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran [see Clinical Pharmacology (12.3)]. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone.
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
Consider reducing the dose of PRADAXA to 75 mg twice daily when dronedarone or systemic ketoconazole is coadministered with PRADAXA in patients with moderate renal impairment (CrCl 30-50 mL/min). Avoid use of PRADAXA and P-gp inhibitors in patients with severe renal impairment (CrCl 15-30 mL/min) [see Drug Interactions (7.1) and Use in Specific Populations (8.6)].
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
Avoid use of PRADAXA and concomitant P-gp inhibitors in patients with CrCl <50 mL/min [see Drug Interactions (7.2) and Use in Specific Populations (8.6)].
6 ADVERSE REACTIONS
The most serious adverse reactions reported with PRADAXA were related to bleeding [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
The RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy) study provided safety information on the use of two doses of PRADAXA and warfarin [see Clinical Studies (14.1)]. The numbers of patients and their exposures are described in Table 1. Limited information is presented on the 110 mg dosing arm because this dose is not approved.
| PRADAXA 110 mg twice daily | PRADAXA 150 mg twice daily | Warfarin | ||
| Total number treated | 5983 | 6059 | 5998 | |
| Exposure | ||||
| > 12 months | 4936 | 4939 | 5193 | |
| > 24 months | 2387 | 2405 | 2470 | |
| Mean exposure (months) | 20.5 | 20.3 | 21.3 | |
| Total patient-years | 10,242 | 10,261 | 10,659 | |
Drug Discontinuation in RE-LY
The rates of adverse reactions leading to treatment discontinuation were 21% for PRADAXA 150 mg and 16% for warfarin. The most frequent adverse reactions leading to discontinuation of PRADAXA were bleeding and gastrointestinal events (i.e., dyspepsia, nausea, upper abdominal pain, gastrointestinal hemorrhage, and diarrhea).
Bleeding [see Warnings and Precautions (5.2)]
Table 2 shows the number of patients experiencing serious bleeding during the treatment period in the RE-LY study, with the bleeding rate per 100 patient-years (%). Major bleeds fulfilled one or more of the following criteria: bleeding associated with a reduction in hemoglobin of at least 2 grams per deciliter or leading to a transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding). A life-threatening bleed met one or more of the following criteria: fatal, symptomatic intracranial bleed, reduction in hemoglobin of at least 5 grams per deciliter, transfusion of at least 4 units of blood, associated with hypotension requiring the use of intravenous inotropic agents, or necessitating surgical intervention. Intracranial hemorrhage included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
| * Patients contributed multiple events and events
were counted in multiple categories. ** Confidence interval |
|||
|
PRADAXA 150 mg twice daily N (%) |
Warfarin N (%) |
Hazard Ratio (95% CI**) |
|
| Randomized patients | 6076 | 6022 | |
| Patient-years | 12,033 | 11,794 | |
| Intracranial hemorrhage | 38 (0.3) | 90 (0.8) | 0.41 (0.28, 0.60) |
| Life-threatening bleed | 179 (1.5) | 218 (1.9) | 0.80 (0.66, 0.98) |
| Major bleed | 399 (3.3) | 421 (3.6) | 0.93 (0.81, 1.07) |
| Any bleed | 1993 (16.6) | 2166 (18.4) | 0.91 (0.85, 0.96) |
The risk of major bleeds was similar with PRADAXA 150 mg and warfarin across major subgroups defined by baseline characteristics, with the exception of age, where there was a trend towards a higher incidence of major bleeding on PRADAXA (hazard ratio 1.2, 95% CI: 1.0 to 1.4) for patients ≥75 years of age.
There was a higher rate of major gastrointestinal bleeds in patients receiving PRADAXA 150 mg than in patients receiving warfarin (1.6% vs. 1.1%, respectively, with a hazard ratio vs. warfarin of 1.5, 95% CI, 1.2 to 1.9), and a higher rate of any gastrointestinal bleeds (6.1% vs. 4.0%, respectively).
Gastrointestinal Adverse Reactions
Patients on PRADAXA 150 mg had an increased incidence of gastrointestinal adverse reactions (35% vs. 24% on warfarin). These were commonly dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) and gastritis-like symptoms (including GERD, esophagitis, erosive gastritis, gastric hemorrhage, hemorrhagic gastritis, hemorrhagic erosive gastritis, and gastrointestinal ulcer).
Hypersensitivity Reactions
In the RE-LY study, drug hypersensitivity (including urticaria, rash, and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were reported in <0.1% of patients receiving PRADAXA.
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
PRADAXA was studied in 4387 patients in 4 pivotal, parallel, randomized, double-blind trials. Three of these trials were active-controlled (warfarin) (RE-COVER, RE-COVER II, and RE-MEDY), and one study (RE-SONATE) was placebo-controlled. The demographic characteristics were similar among the 4 pivotal studies and between the treatment groups within these studies. Approximately 60% of the treated patients were male, with a mean age of 55.1 years. The majority of the patients were white (87.7%), 10.3% were Asian, and 1.9% were black with a mean CrCl of 105.6 mL/min.
Bleeding events for the 4 pivotal studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2.0 g/dL (1.24 mmol/L or more, or leading to transfusion of 2 or more units of whole blood or red cells).
RE-COVER and RE-COVER II studies compared PRADAXA 150 mg twice daily and warfarin for the treatment of deep vein thrombosis and pulmonary embolism. Patients received 5-10 days of an approved parenteral anticoagulant therapy followed by 6 months, with mean exposure of 164 days, of oral only treatment; warfarin was overlapped with parenteral therapy. Table 3 shows the number of patients experiencing bleeding events in the pooled analysis of RE-COVER and RE-COVER II studies during the full treatment including parenteral and oral only treatment periods after randomization.
| Note: MBE can belong to more than one criterion. aPatients with at least one MBE. bBleeding site based on investigator assessment. Patients can have more than one site of bleeding. cConfidence interval |
||||
|
Bleeding Events-Full
Treatment Period Including Parenteral Treatment |
||||
|
PRADAXA 150 mg twice daily N (%) |
Warfarin N (%) |
Hazard Ratio (95% CI)c |
||
| Patients | N=2553 | N=2554 | ||
| Major bleeding eventa | 37 (1.4) | 51 (2.0) | 0.73 ( 0.48, 1.11) | |
| Fatal bleeding | 1 (0.04) | 2 (0.1) | ||
| Bleeding in a critical area or organ | 7 (0.3) | 15 (0.6) | ||
| Fall in hemoglobin ≥2g/dL or transfusion ≥2 units of whole blood or packed red blood cells | 32 (1.3) | 38 (1.5) | ||
| Bleeding sites for MBEb | ||||
| Intracranial | 2 (0.1) | 5 (0.2) | ||
| Retroperitoneal | 2 (0.1) | 1 (0.04) | ||
| Intraarticular | 2 (0.1) | 4 (0.2) | ||
| Intramuscular | 2 (0.1) | 6 (0.2) | ||
| Gastrointestinal | 15 (0.6) | 14 (0.5) | ||
| Urogenital | 7 (0.3) | 14 (0.5) | ||
| Other | 8 (0.3) | 8 (0.3) | ||
| Clinically relevant non-major bleeding | 101 (4.0) | 170 (6.7) | 0.58 (0.46, 0.75) | |
| Any bleeding | 411 (16.1) | 567 (22.7) | 0.70 (0.61, 0.79) | |
The rate of any gastrointestinal bleeds in patients receiving PRADAXA 150 mg in the full treatment period was 3.1% (2.4% on warfarin).
The RE-MEDY and RE-SONATE studies provided safety information on the use of PRADAXA for the reduction in the risk of recurrence of deep vein thrombosis and pulmonary embolism.
RE-MEDY was an active-controlled study (warfarin) in which 1430 patients received PRADAXA 150 mg twice daily following 6 to 18 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-MEDY study had a combined treatment duration of up to more than 3 years, with mean exposure of 473 days. Table 4 shows the number of patients experiencing bleeding events in the study.
| Note: MBE can belong to more than one criterion. aPatients with at least one MBE. bBleeding site based on investigator assessment. Patients can have more than one site of bleeding. cConfidence interval |
||||
|
PRADAXA 150 mg twice daily N (%) |
Warfarin N (%) |
Hazard Ratio (95% CI)c |
||
| Patients | N=1430 | N=1426 | ||
| Major bleeding eventa | 13 (0.9) | 25 (1.8) | 0.54 (0.25, 1.16) | |
| Fatal bleeding | 0 | 1 (0.1) | ||
| Bleeding in a critical area or organ | 7 (0.5) | 11 (0.8) | ||
| Fall in hemoglobin ≥2g/dL or transfusion ≥2 units of whole blood or packed red blood cells | 7 (0.5) | 16 (1.1) | ||
| Bleeding sites for MBEb | ||||
| Intracranial | 2 (0.1) | 4 (0.3) | ||
| Intraocular | 4 (0.3) | 2 (0.1) | ||
| Retroperitoneal | 0 | 1 (0.1) | ||
| Intraarticular | 0 | 2 (0.1) | ||
| Intramuscular | 0 | 4 (0.3) | ||
| Gastrointestinal | 4 (0.3) | 8 (0.6) | ||
| Urogenital | 1 (0.1) | 1 (0.1) | ||
| Other | 2 (0.1) | 4 (0.3) | ||
| Clinically relevant non-major bleeding | 71 (5.0) | 125 (8.8) | 0.56 (0.42, 0.75) | |
| Any bleeding | 278 (19.4) | 373 (26.2) | 0.71 (0.61, 0.83) | |
In the RE-MEDY study, the rate of any gastrointestinal bleeds in patients receiving PRADAXA 150 mg was 3.1% (2.2% on warfarin).
RE-SONATE was a placebo-controlled study in which 684 patients received PRADAXA 150 mg twice daily following 3 to 6 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-SONATE study had combined treatment duration up to 9 months, with mean exposure of 165 days. Table 5 shows the number of patients experiencing bleeding events in the study.
| Note: MBE can belong to more than one criterion. aPatients with at least one MBE. bBleeding site based on investigator assessment. Patients can have more than one site of bleeding. cConfidence interval |
||||
|
PRADAXA 150 mg twice daily N (%) |
Placebo N (%) |
Hazard Ratio (95% CI)c |
||
| Patients | N=684 | N=659 | ||
| Major bleeding eventa | 2 (0.3) | 0 | ||
| Bleeding in a critical area or organ | 2 (0.3) | 0 | ||
| Gastrointestinalb | 2 (0.3) | 0 | ||
| Clinically relevant non-major bleeding | 34 (5.0) | 13 (2.0) | 2.54 (1.34, 4.82) | |
| Any bleeding | 72 (10.5) | 40 (6.1) | 1.77 (1.20, 2.61) | |
In the RE-SONATE study, the rate of any gastrointestinal bleeds in patients receiving PRADAXA 150 mg was 0.7% (0.3% on placebo).
Clinical Myocardial Infarction Events
In the active-controlled VTE studies, a higher rate of
clinical myocardial infarction was reported in patients who received
PRADAXA [20 (0.66 per 100 patient-years)] than in those who received
warfarin [5 (0.17 per 100 patient-years)]. In the placebo-controlled
study, a similar rate of non-fatal and fatal clinical myocardial infarction
was reported in patients who received PRADAXA [1 (0.32 per 100 patient-years)]
and in those who received placebo [1 (0.34 per 100 patient-years)].
Gastrointestinal Adverse
Reactions
In the four pivotal studies, patients
on PRADAXA 150 mg had a similar incidence of gastrointestinal adverse
reactions (24.7% vs. 22.7% on warfarin). Dyspepsia (including abdominal
pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort)
occurred in patients on PRADAXA in 7.5% vs. 5.5% on warfarin, and
gastritis-like symptoms (including gastritis, GERD, esophagitis, erosive
gastritis and gastric hemorrhage) occurred at 3.0% vs. 1.7%, respectively.
Hypersensitivity Reactions
In the 4 pivotal studies, drug hypersensitivity (including
urticaria, rash, and pruritus), allergic edema, anaphylactic reaction,
and anaphylactic shock were reported in 0.1% of patients receiving
PRADAXA.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of PRADAXA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been identified during post approval use of PRADAXA: angioedema, thrombocytopenia, esophageal ulcer.
7 DRUG INTERACTIONS
7.1 Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
The concomitant use of PRADAXA with P-gp inducers (e.g., rifampin) reduces exposure to dabigatran and should generally be avoided [see Clinical Pharmacology (12.3)].
P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran [see Clinical Pharmacology (12.3)]. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone.
In patients with moderate renal impairment (CrCl 30-50 mL/min), consider reducing the dose of PRADAXA to 75 mg twice daily when administered concomitantly with the P-gp inhibitor dronedarone or systemic ketoconazole. The use of P-gp inhibitors (verapamil, amiodarone, quinidine, and clarithromycin) does not require a dose adjustment of PRADAXA. These results should not be extrapolated to other P-gp inhibitors [see Warnings and Precautions (5.4), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
The concomitant use of PRADAXA and P-gp inhibitors in patients with severe renal impairment (CrCl 15-30 mL/min) should be avoided [see Warnings and Precautions (5.5), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
7.2 Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
Avoid use of PRADAXA and P-gp inhibitors in patients with CrCl <50 mL/min [see Warnings and Precautions (5.5), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women.
Dabigatran has been shown to decrease the number of implantations when male and female rats were treated at a dosage of 70 mg/kg (about 2.6 to 3.0 times the human exposure at maximum recommended human dose [MRHD] of 300 mg/day based on area under the curve [AUC] comparisons) prior to mating and up to implantation (gestation Day 6). Treatment of pregnant rats after implantation with dabigatran at the same dose increased the number of dead offspring and caused excess vaginal/uterine bleeding close to parturition. Although dabigatran increased the incidence of delayed or irregular ossification of fetal skull bones and vertebrae in the rat, it did not induce major malformations in rats or rabbits.
8.2 Labor and Delivery
Safety and effectiveness of PRADAXA during labor and delivery have not been studied in clinical trials. Consider the risks of bleeding and of stroke in using PRADAXA in this setting [see Warnings and Precautions (5.2)].
Death of offspring and mother rats during labor in association with uterine bleeding occurred during treatment of pregnant rats from implantation (gestation Day 7) to weaning (lactation Day 21) with dabigatran at a dose of 70 mg/kg (about 2.6 times the human exposure at MRHD of 300 mg/day based on AUC comparisons).
8.3 Nursing Mothers
It is not known whether dabigatran is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from PRADAXA, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of PRADAXA in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients in the RE-LY study, 82% were 65 and over, while 40% were 75 and over. The risk of stroke and bleeding increases with age, but the risk-benefit profile is favorable in all age groups [see Warnings and Precautions (5), Adverse Reactions (6.1), and Clinical Studies (14.1)].
8.6 Renal Impairment
Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
No dose adjustment of PRADAXA is recommended in patients with mild or moderate renal impairment [see Clinical Pharmacology (12.3)]. Reduce the dose of PRADAXA in patients with severe renal impairment (CrCl 15-30 mL/min) [see Dosage and Administration (2.1, 2.2) and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with CrCl <15 mL/min or on dialysis cannot be provided.
Adjust dose appropriately in patients with renal impairment receiving concomitant P-gp inhibitors [see Warnings and Precautions (5.5), Drug Interactions (7.1), and Clinical Pharmacology (12.3)].
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary Embolism
Patients with severe renal impairment (CrCL <30 mL/min) were excluded from RE-COVER.
Dosing recommendations for patients with CrCl <30 mL/min or on dialysis cannot be provided. Avoid use of PRADAXA with concomitant P-gp inhibitors in patients with CrCl <50 mL/min [see Warnings and Precautions (5.5), Drug Interactions (7.2), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Accidental overdose may lead to hemorrhagic complications. There is no reversal agent for dabigatran. In the event of hemorrhagic complications, initiate appropriate clinical support, discontinue treatment with PRADAXA, and investigate the source of bleeding. Dabigatran is primarily eliminated by the kidneys with a low plasma protein binding of approximately 35%. Hemodialysis can remove dabigatran; however, data supporting this approach are limited. Using a high-flux dialyzer, blood flow rate of 200 mL/min, and dialysate flow rate of 700 mL/min, approximately 49% of total dabigatran can be cleared from plasma over 4 hours. At the same dialysate flow rate, approximately 57% can be cleared using a dialyzer blood flow rate of 300 mL/min, with no appreciable increase in clearance observed at higher blood flow rates. Upon cessation of hemodialysis, a redistribution effect of approximately 7% to 15% is seen. The effect of dialysis on dabigatran’s plasma concentration would be expected to vary based on patient specific characteristics. Measurement of aPTT or ECT may help guide therapy [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)].
11 DESCRIPTION
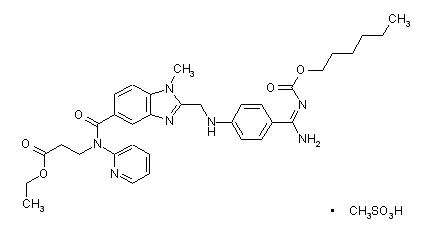
The chemical name for dabigatran etexilate mesylate, a direct thrombin inhibitor, is β-Alanine, N-[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl] phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-,ethyl ester, methanesulfonate. The empirical formula is C34H41N7O5 ⋅ CH4O3S and the molecular weight is 723.86 (mesylate salt), 627.75 (free base). The structural formula is:
Dabigatran etexilate mesylate is a yellow-white to yellow powder. A saturated solution in pure water has a solubility of 1.8 mg/mL. It is freely soluble in methanol, slightly soluble in ethanol, and sparingly soluble in isopropanol.
The 150 mg capsule for oral administration contains 172.95 mg dabigatran etexilate mesylate, which is equivalent to 150 mg of dabigatran etexilate, and the following inactive ingredients: acacia, dimethicone, hypromellose, hydroxypropyl cellulose, talc, and tartaric acid. The capsule shell is composed of carrageenan, FD&C Blue No. 2 (150 mg only), FD&C Yellow No. 6, hypromellose, potassium chloride, titanium dioxide, and black edible ink. The 75 mg capsule contains 86.48 mg dabigatran etexilate mesylate, equivalent to 75 mg dabigatran etexilate, and is otherwise similar to the 150 mg capsule.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dabigatran and its acyl glucuronides are competitive, direct thrombin inhibitors. Because thrombin (serine protease) enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents the development of a thrombus. Both free and clot-bound thrombin, and thrombin-induced platelet aggregation are inhibited by the active moieties.
12.2 Pharmacodynamics
At recommended therapeutic doses, dabigatran etexilate prolongs the coagulation markers such as aPTT, ECT, and TT. INR is relatively insensitive to the exposure to dabigatran and cannot be interpreted the same way as used for warfarin monitoring.
The aPTT test provides an approximation of PRADAXA’s anticoagulant effect. The average time course for effects on aPTT, following approved dosing regimens in patients with various degrees of renal impairment is shown in Figure 1. The curves represent mean levels without confidence intervals; variations should be expected when measuring aPTT. While advice cannot be provided on the level of recovery of aPTT needed in any particular clinical setting, the curves can be used to estimate the time to get to a particular level of recovery, even when the time since the last dose of PRADAXA is not precisely known. In the RE-LY trial, the median (10th to 90th percentile) trough aPTT in patients receiving the 150 mg dose was 52 (40 to 76) seconds.
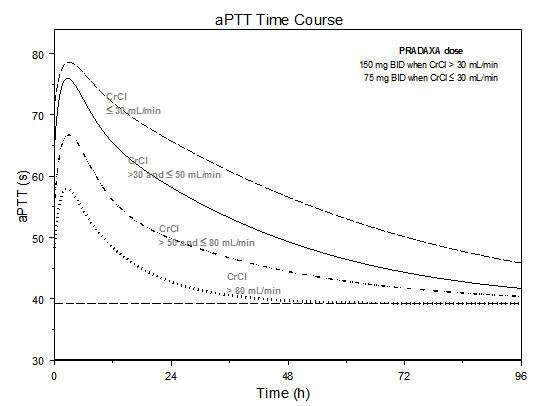
*Simulations based on PK data from a study in subjects with renal impairment and PK/aPTT relationships derived from the RE-LY study; aPTT prolongation in RE-LY was measured centrally in citrate plasma using PTT Reagent Roche Diagnostics GmbH, Mannheim, Germany. There may be quantitative differences between various established methods for aPTT assessment.
The degree of anticoagulant activity can also be assessed by the ecarin clotting time (ECT). This test is a more specific measure of the effect of dabigatran than activated partial thromboplastin time (aPTT). In the RE-LY trial, the median (10th to 90th percentile) trough ECT in patients receiving the 150 mg dose was 63 (44 to 103) seconds.
Cardiac Electrophysiology
No prolongation of the QTc interval was observed with dabigatran etexilate at doses up to 600 mg.
12.3 Pharmacokinetics
Dabigatran etexilate mesylate is absorbed as the dabigatran etexilate ester. The ester is then hydrolyzed, forming dabigatran, the active moiety. Dabigatran is metabolized to four different acyl glucuronides and both the glucuronides and dabigatran have similar pharmacological activity. Pharmacokinetics described here refer to the sum of dabigatran and its glucuronides. Dabigatran displays dose-proportional pharmacokinetics in healthy subjects and patients in the range of doses from 10 to 400 mg.
Absorption
The absolute bioavailability of dabigatran following oral administration of dabigatran etexilate is approximately 3 to 7%. Dabigatran etexilate is a substrate of the efflux transporter P-gp. After oral administration of dabigatran etexilate in healthy volunteers, Cmax occurs at 1 hour post-administration in the fasted state. Coadministration of PRADAXA with a high-fat meal delays the time to Cmax by approximately 2 hours but has no effect on the bioavailability of dabigatran; PRADAXA may be administered with or without food.
The oral bioavailability of dabigatran etexilate increases by 75% when the pellets are taken without the capsule shell compared to the intact capsule formulation. PRADAXA capsules should therefore not be broken, chewed, or opened before administration.
Distribution
Dabigatran is approximately 35% bound to human plasma proteins. The red blood cell to plasma partitioning of dabigatran measured as total radioactivity is less than 0.3. The volume of distribution of dabigatran is 50 to 70 L. Dabigatran pharmacokinetics are dose proportional after single doses of 10 to 400 mg. Given twice daily, dabigatran’s accumulation factor is approximately two.
Elimination
Dabigatran is eliminated primarily in the urine. Renal clearance of dabigatran is 80% of total clearance after intravenous administration. After oral administration of radiolabeled dabigatran, 7% of radioactivity is recovered in urine and 86% in feces. The half-life of dabigatran in healthy subjects is 12 to 17 hours.
Metabolism
After oral administration, dabigatran etexilate is converted to dabigatran. The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to the active principal dabigatran is the predominant metabolic reaction. Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes. Dabigatran is subject to conjugation forming pharmacologically active acyl glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide exist, and each accounts for less than 10% of total dabigatran in plasma.
Renal Impairment
An open, parallel-group single-center study compared dabigatran pharmacokinetics in healthy subjects and patients with mild to moderate renal impairment receiving a single dose of PRADAXA 150 mg. Exposure to dabigatran increases with severity of renal function impairment (Table 6). Similar findings were observed in the RE-LY and RE-COVER trials.
| +Patients with severe renal impairment were not studied in RE-LY and RE-COVER. Dosing recommendations in subjects with severe renal impairment are based on pharmacokinetic modeling [see Dosage and Administration (2.1, 2.2) and Use in Specific Populations (8.6)]. | ||||
| Renal Function | CrCl (mL/min) | Increase in AUC | Increase in Cmax |
t1/2
(h) |
| Normal | ≥ 80 | 1x | 1x | 13 |
| Mild | 50-80 | 1.5x | 1.1x | 15 |
| Moderate | 30-50 | 3.2x | 1.7x | 18 |
| Severe+ | 15-30 | 6.3x | 2.1x | 27 |
Hepatic Impairment
Administration of PRADAXA in patients with moderate hepatic impairment (Child-Pugh B) showed a large inter-subject variability, but no evidence of a consistent change in exposure or pharmacodynamics.
Drug Interactions
Impact of Other Drugs
on Dabigatran
P-gp Inducers
Rifampin: Rifampin 600 mg once daily for 7 days followed by a single dose of dabigatran decreased its AUC and Cmax by 66% and 67%, respectively. By Day 7 after cessation of rifampin treatment, dabigatran exposure was close to normal [see Warnings and Precautions (5.4) and Drug Interactions (7)].
P-gp Inhibitors
In studies with the P-gp inhibitors ketoconazole, amiodarone, verapamil, and quinidine, the time to peak, terminal half-life, and mean residence time of dabigatran were not affected. Any observed changes in Cmax and AUC are described below.
Dronedarone: Simultaneous administration of dabigatran etexilate and dronedarone (administered once or twice daily) increases exposure to dabigatran by 70 to 140% compared to dabigatran alone. The increase in exposure is only 30 to 60% higher compared to dabigatran alone when dronedarone is administered 2 hours after dabigatran etexilate.
Ketoconazole: Systemic ketoconazole increased dabigatran AUC and Cmax values by 138% and 135%, respectively, after a single dose of 400 mg, and 153%, and 149%, respectively, after multiple daily doses of 400 mg.
Verapamil: When dabigatran etexilate was coadministered with oral verapamil, the Cmax and AUC of dabigatran were increased. The extent of increase depends on the formulation of verapamil and timing of administration. If verapamil is present in the gut when dabigatran is taken, it will increase exposure to dabigatran with the greatest increase observed when a single dose of immediate-release verapamil is given one hour prior to dabigatran (AUC increased by a factor of 2.4). If verapamil is given 2 hours after dabigatran, the increase in AUC is negligible. In the population pharmacokinetics study from RE-LY, no important changes in dabigatran trough levels were observed in patients who received verapamil. Similar findings were observed in the RE-COVER study.
Amiodarone: When dabigatran etexilate was coadministered with a single 600 mg oral dose of amiodarone, the dabigatran AUC and Cmax increased by 58% and 50%, respectively. The increase in exposure was mitigated by a 65% increase in the renal clearance of dabigatran in the presence of amiodarone. The increase in renal clearance may persist after amiodarone is discontinued because of amiodarone’s long half-life. In the population pharmacokinetics study from RE-LY, no important changes in dabigatran trough levels were observed in patients who received amiodarone.
Quinidine: Quinidine was given as a 200 mg dose every 2 hours up to a total dose of 1000 mg. Dabigatran etexilate was given over 3 consecutive days, the last evening dose on Day 3 with or without quinidine pre-dosing. Concomitant quinidine administration increased dabigatran’s AUC and Cmax by 53% and 56%, respectively.
Clarithromycin: Coadministered clarithromycin had no impact on the exposure to dabigatran.
Other Drugs
Clopidogrel: When dabigatran etexilate was given concomitantly with a loading dose of 300 mg or 600 mg clopidogrel, the dabigatran AUC and Cmax increased by approximately 30% and 40%, respectively. The concomitant administration of dabigatran etexilate and clopidogrel resulted in no further prolongation of capillary bleeding times compared to clopidogrel monotherapy. When comparing combined treatment and the respective mono-treatments, the coagulation measures for dabigatran’s effect (aPTT, ECT, and TT) remained unchanged, and inhibition of platelet aggregation (IPA), a measurement of clopidogrel’s effect, remained unchanged.
Enoxaparin: Enoxaparin 40 mg given subcutaneously for 3 days with the last dose given 24 hours before a single dose of PRADAXA had no impact on the exposure to dabigatran or the coagulation measures aPTT, ECT, or TT.
Diclofenac, Ranitidine, and Digoxin: None of these drugs alters exposure to dabigatran.
In RE-LY, dabigatran plasma samples were also collected. The concomitant use of proton pump inhibitors, H2 antagonists, and digoxin did not appreciably change the trough concentration of dabigatran.
Impact of Dabigatran on Other Drugs
In clinical studies exploring CYP3A4, CYP2C9, P-gp and
other pathways, dabigatran did not meaningfully alter the pharmacokinetics
of amiodarone, atorvastatin, clarithromycin, diclofenac, clopidogrel,
digoxin, pantoprazole, or ranitidine.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dabigatran was not carcinogenic when administered by oral gavage to mice and rats for up to 2 years. The highest doses tested (200 mg/kg/day) in mice and rats were approximately 3.6 and 6 times, respectively, the human exposure at MRHD of 300 mg/day based on AUC comparisons.
Dabigatran was not mutagenic in in vitro tests, including bacterial reversion tests, mouse lymphoma assay and chromosomal aberration assay in human lymphocytes, and the in vivo micronucleus assay in rats.
In the rat fertility study with oral gavage doses of 15, 70, and 200 mg/kg, males were treated for 29 days prior to mating, during mating up to scheduled termination, and females were treated 15 days prior to mating through gestation Day 6. No adverse effects on male or female fertility were observed at 200 mg/kg or 9 to 12 times the human exposure at MRHD of 300 mg/day based on AUC comparisons. However, the number of implantations decreased in females receiving 70 mg/kg, or 3 times the human exposure at MRHD based on AUC comparisons.
14 CLINICAL STUDIES
14.1 Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation
The clinical evidence for the efficacy of PRADAXA was derived from RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy), a multi-center, multi-national, randomized parallel group trial comparing two blinded doses of PRADAXA (110 mg twice daily and 150 mg twice daily) with open-label warfarin (dosed to target INR of 2 to 3) in patients with non-valvular, persistent, paroxysmal, or permanent atrial fibrillation and one or more of the following additional risk factors:
- Previous stroke, transient ischemic attack (TIA), or systemic embolism
- Left ventricular ejection fraction <40%
- Symptomatic heart failure, ≥ New York Heart Association Class 2
- Age ≥75 years
- Age ≥65 years and one of the following: diabetes mellitus, coronary artery disease (CAD), or hypertension
The primary objective of this study was to determine if PRADAXA was non-inferior to warfarin in reducing the occurrence of the composite endpoint, stroke (ischemic and hemorrhagic) and systemic embolism. The study was designed to ensure that PRADAXA preserved more than 50% of warfarin’s effect as established by previous randomized, placebo-controlled trials of warfarin in atrial fibrillation. Statistical superiority was also analyzed.
A total of 18,113 patients were randomized and followed for a median of 2 years. The patients’ mean age was 71.5 years and the mean CHADS2 score was 2.1. The patient population was 64% male, 70% Caucasian, 16% Asian, and 1% black. Twenty percent of patients had a history of a stroke or TIA and 50% were Vitamin K antagonist (VKA) naïve, defined as less than 2 months total lifetime exposure to a VKA. Thirty-two percent of the population had never been exposed to a VKA. Concomitant diseases of patients in this trial included hypertension 79%, diabetes 23%, and CAD 28%. At baseline, 40% of patients were on aspirin and 6% were on clopidogrel. For patients randomized to warfarin, the mean percentage of time in therapeutic range (INR 2 to 3) was 64%.
Relative to warfarin and to PRADAXA 110 mg twice daily, PRADAXA 150 mg twice daily significantly reduced the primary composite endpoint of stroke and systemic embolism (see Table 7 and Figure 2).
|
PRADAXA 150 mg twice daily |
PRADAXA 110 mg twice daily |
Warfarin | |
| Patients randomized | 6076 | 6015 | 6022 |
| Patients (%) with events | 134 (2.2%) | 183 (3%) | 202 (3.4%) |
| Hazard ratio vs. warfarin (95% CI) | 0.65 (0.52, 0.81) | 0.90 (0.74, 1.10) | |
| P-value for superiority | 0.0001 | 0.3 | |
| Hazard ratio vs. PRADAXA 110 mg (95% CI) | 0.72 (0.58, 0.90) | ||
| P-value for superiority | 0.004 |
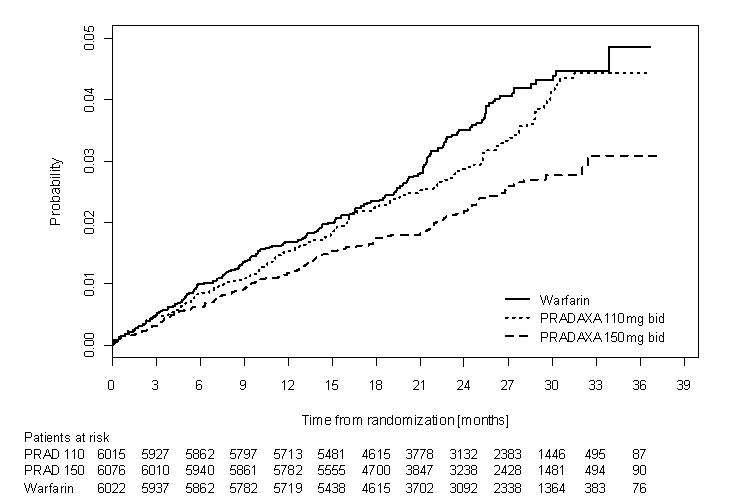
The contributions of the components of the composite endpoint, including stroke by subtype, are shown in Table 8. The treatment effect was primarily a reduction in stroke. PRADAXA 150 mg twice daily was superior in reducing ischemic and hemorrhagic strokes relative to warfarin.
|
PRADAXA 150 mg twice daily |
Warfarin |
Hazard ratio
vs. warfarin
(95% CI) |
|
| Patients randomized | 6076 | 6022 | |
| Stroke | 122 | 186 | 0.64 (0.51, 0.81) |
| Ischemic stroke | 103 | 134 | 0.75 (0.58, 0.97) |
| Hemorrhagic stroke | 12 | 45 | 0.26 (0.14, 0.49) |
| Systemic embolism | 13 | 21 | 0.61 (0.30, 1.21) |
In the RE-LY trial, the rate of all-cause mortality was lower on dabigatran 150 mg than on warfarin (3.6% per year versus 4.1% per year). The rate of vascular death was lower on dabigatran 150 mg compared to warfarin (2.3% per year versus 2.7% per year). Non-vascular death rates were similar in the treatment arms.
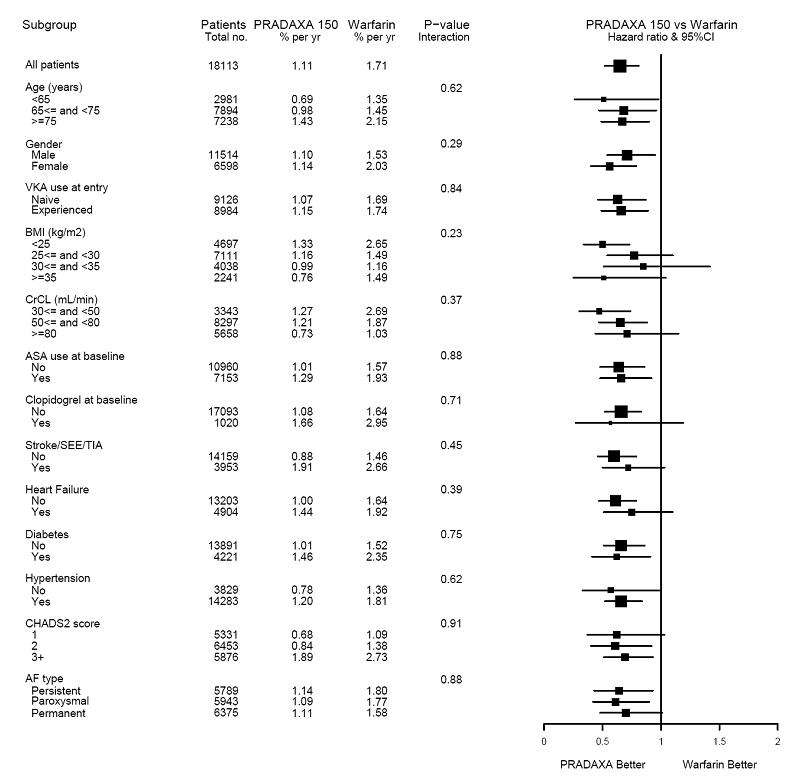
The efficacy of PRADAXA 150 mg twice daily was generally consistent across major subgroups (see Figure 3).
In RE-LY, a higher rate of clinical myocardial infarction was reported in patients who received PRADAXA (0.7 per 100 patient-years for 150 mg dose) than in those who received warfarin (0.6).
14.2 Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis (DVT) and Pulmonary Embolism (PE)
In the randomized, parallel group, double-blind trials, RE-COVER and RE-COVER II, patients with deep vein thrombosis and pulmonary embolism received PRADAXA 150 mg twice daily or warfarin (dosed to target INR of 2 to 3) following initial treatment with an approved parenteral anticoagulant for 5-10 days.
In RE-COVER, the median treatment duration during the oral only treatment period was 174 days. A total of 2539 patients (30.9% patients with symptomatic PE with or without DVT and 68.9% with symptomatic DVT only) were treated with a mean age of 54.7 years. The patient population was 58.4% male, 94.8% white, 2.6% Asian, and 2.6% black. The concomitant diseases of patients in this trial included hypertension (35.9%), diabetes mellitus (8.3%), coronary artery disease (6.5%), active cancer (4.8%), and gastric or duodenal ulcer (4.4%). Concomitant medications included agents acting on renin-angiotensin system (25.2%), vasodilators (28.4%), serum lipid-reducing agents (18.2%), NSAIDs (21%), beta-blockers (14.8%), calcium channel blockers (8.5%), ASA (8.6%), and platelet inhibitors excluding ASA (0.6%). Patients randomized to warfarin had a mean percentage of time in the INR target range of 2.0 to 3.0 of 60% in RE-COVER study.
In RE-COVER II, the median treatment duration during the oral only treatment period was 174 days. A total of 2568 patients (31.8% patients with symptomatic PE with or without DVT and 68.1% with symptomatic DVT only) were treated with a mean age of 54.9 years. The patient population was 60.6% male, 77.6% white, 20.9% Asian, and 1.5% black. The concomitant diseases of patients in this trial included hypertension (35.1%), diabetes mellitus (9.8%), coronary artery disease (7.1%), active cancer (3.9%), and gastric or duodenal ulcer (3.8%). Concomitant medications included agents acting on renin-angiotensin system (24.2%), vasodilators (28.6%), serum lipid-reducing agents (20.0%), NSAIDs (22.3%), beta-blockers (14.8%), calcium channel blockers (10.8%), ASA (9.8%), and platelet inhibitors excluding ASA (0.8%). Patients randomized to warfarin had a mean percentage of time in the INR target range of 2.0 to 3.0 of 57% in RE-COVER II study.
In studies RE-COVER and RE-COVER II, the protocol specified non-inferiority margin (2.75) for the hazard ratio was derived based on the upper limit of the 95% confidence interval of the historical warfarin effect. PRADAXA was demonstrated to be non-inferior to warfarin (dosed to target INR of 2 to 3) (Table 9) based on the primary composite endpoint (fatal PE or symptomatic non-fatal PE and/or DVT) and retains at least 66.9% (RE-COVER) and 63.9% (RE-COVER II) of the historical warfarin effect respectively.
|
aModified ITT analyses
population consists of all randomized patients who received at least
one dose of study medication. bNumber of patients with one or more event. cNumber of events. For patients with multiple events each event is counted independently. |
||||
|
PRADAXA 150 mg twice daily N (%) |
Warfarin N (%) |
Hazard Ratio vs. warfarin (95% CI) |
||
| RE-COVER | N=1274 | N=1265 | ||
| Primary Composite Endpointb | 34 (2.7) | 32 (2.5) | 1.05 (0.65, 1.70) | |
| Fatal PEc | 1 (0.1) | 3 (0.2) | ||
| Symptomatic non-fatal PEc | 16 (1.3) | 8 (0.6) | ||
| Symptomatic recurrent DVTc | 17 (1.3) | 23 (1.8) | ||
| RE-COVER II | N=1279 | N=1289 | ||
| Primary Composite Endpointb | 34 (2.7) | 30 (2.3) | 1.13 (0.69, 1.85) | |
| Fatal PEc | 3 (0.2) | 0 | ||
| Symptomatic non-fatal PEc | 9 (0.7) | 15 (1.2) | ||
| Symptomatic recurrent DVTc | 30 (2.3) | 17 (1.3) | ||
In the randomized, parallel group, double-blind, pivotal trial, RE-MEDY, patients received PRADAXA 150 mg twice daily or warfarin (dosed to target INR of 2 to 3) following 3 to 12 months of treatment with anticoagulation therapy for an acute VTE. The median treatment duration during the oral only treatment period was 534 days. A total of 2856 patients were treated with a mean age of 54.6 years. The patient population was 61% male, and 90.1% white, 7.9% Asian and 2.0% black. The concomitant diseases of patients in this trial included hypertension (38.6%), diabetes mellitus (9.0%), coronary artery disease (7.2%), active cancer (4.2%), and gastric or duodenal ulcer (3.8%). Concomitant medications included agents acting on renin-angiotensin system (27.9%), vasodilators (26.7%), serum lipid reducing agents (20.6%), NSAIDs (18.3%), beta-blockers (16.3%), calcium channel blockers (11.1%), aspirin (7.7%), and platelet inhibitors excluding ASA (0.9%). Patients randomized to warfarin had a mean percentage of time in the INR target range of 2.0 to 3.0 of 62% in the study.
In study RE-MEDY, the protocol specified non-inferiority margin (2.85) for the hazard ratio was derived based on the point estimate of the historical warfarin effect. PRADAXA was demonstrated to be non-inferior to warfarin (dosed to target INR of 2 to 3) (Table 10) based on the primary composite endpoint (fatal PE or symptomatic non-fatal PE and/or DVT) and retains at least 63.0% of the historical warfarin effect. If the non-inferiority margin was derived based on the 50% retention of the upper limit of the 95% confidence interval, PRADAXA was demonstrated to retain at least 33.4% of the historical warfarin effect based on the composite primary endpoint.
|
aModified ITT analyses
population consists of all randomized patients who received at least
one dose of study medication. bNumber of patients with one or more event. cNumber of events. For patients with multiple events each event is counted independently. |
||||
|
PRADAXA 150 mg twice daily N=1430 N (%) |
Warfarin N=1426 N (%) |
Hazard Ratio vs. warfarin (95% CI) |
||
| Primary Composite Endpointb | 26 (1.8) | 18 (1.3) | 1.44 (0.78, 2.64) | |
| Fatal PEc | 1 (0.07) | 1 (0.07) | ||
| Symptomatic non-fatal PEc | 10 (0.7) | 5 (0.4) | ||
| Symptomatic recurrent DVTc | 17 (1.2) | 13 (0.9) | ||
In a randomized, parallel group, double-blind, pivotal trial, RE-SONATE, patients received PRADAXA 150 mg twice daily or placebo following 6 to 18 months of treatment with anticoagulation therapy for an acute VTE. The median treatment duration was 182 days. A total of 1343 patients were treated with a mean age of 55.8 years. The patient population was 55.5% male, 89.0% white, 9.3% Asian, and 1.7% black. The concomitant diseases of patients in this trial included hypertension (38.8%), diabetes mellitus (8.0%), coronary artery disease (6.0%), active cancer (6.0%), gastric or duodenal ulcer (4.5%), and heart failure (4.6%). Concomitant medications included agents acting on renin-angiotensin system (28.7%), vasodilators (19.4%), beta-blockers (18.5%), serum lipid reducing agents (17.9%), NSAIDs (12.1%), calcium channel blockers (8.9%), aspirin (8.3%), and platelet inhibitors excluding ASA (0.7%). Based on the outcome of the primary composite endpoint (fatal PE, unexplained death, or symptomatic non-fatal PE and/or DVT), PRADAXA was superior to placebo (Table 11).
|
aModified ITT analyses
population consists of all randomized patients who received at least
one dose of study medication. bNumber of patients with one or more events. cNumber of events. For patients with multiple events each event is counted independently. |
||||
|
PRADAXA 150 mg twice daily N=681 N (%) |
Placebo N=662 N (%) |
Hazard Ratio vs. placebo (95% CI) |
||
| Primary Composite Endpointb | 3 (0.4) | 37 (5.6) | 0.08 (0.02, 0.25) p-value <0.0001 |
|
| Fatal PE and unexplained deathc | 0 | 2 (0.3) | ||
| Symptomatic non-fatal PEc | 1 (0.1) | 14 (2.1) | ||
| Symptomatic recurrent DVTc | 2 (0.3) | 23 (3.5) | ||
16 HOW SUPPLIED/STORAGE AND HANDLING
PRADAXA 75 mg capsules have a cream-colored opaque cap imprinted with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted with "R75". The color of the imprinting is black. The capsules are supplied in the packages listed:
- NDC 0597-0149-54 Unit of use bottle of 60 capsules
- NDC 0597-0149-60 Blister package containing 60 capsules (10 x 6 capsule blister cards)
PRADAXA 150 mg capsules have a light blue opaque cap imprinted with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted with "R150". The color of the imprinting is black. The capsules are supplied in the packages listed:
- NDC 0597-0135-54 Unit of use bottle of 60 capsules
- NDC 0597-0135-60 Blister package containing 60 capsules (10 x 6 capsule blister cards)
Bottles
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Once opened, the product must be used within 4 months. Keep the bottle tightly closed. Store in the original package to protect from moisture.
Blisters
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Store in the original package to protect from moisture.
Keep out of the reach of children.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
17.1 Instructions for Patients
- Tell patients to take PRADAXA exactly as prescribed.
- Remind patients not to discontinue PRADAXA without talking to the health care provider who prescribed it.
- Keep PRADAXA in the original bottle to protect from moisture. Do not put PRADAXA in pill boxes or pill organizers.
- When more than one bottle is dispensed to the patient, instruct them to open only one bottle at a time.
- Instruct patient to remove only one capsule from the opened bottle at the time of use. The bottle should be immediately and tightly closed.
- Advise patients not to chew or break the capsules before swallowing them and not to open the capsules and take the pellets alone.
- Advise patients that the capsule should be taken with a full glass of water.
17.2 Bleeding
Inform patients that they may bleed more easily, may bleed longer, and should call their health care provider for any signs or symptoms of bleeding.
Instruct patients to seek emergency care right away if they have any of the following, which may be a sign or symptom of serious bleeding:
- Unusual bruising (bruises that appear without known cause or that get bigger)
- Pink or brown urine
- Red or black, tarry stools
- Coughing up blood
- Vomiting blood, or vomit that looks like coffee grounds
Instruct patients to call their health care provider or to get prompt medical attention if they experience any signs or symptoms of bleeding:
- Pain, swelling or discomfort in a joint
- Headaches, dizziness, or weakness
- Reoccurring nose bleeds
- Unusual bleeding from gums
- Bleeding from a cut that takes a long time to stop
- Menstrual bleeding or vaginal bleeding that is heavier than normal
If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs or platelet inhibitors, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately [see Boxed Warning].
17.3 Gastrointestinal Side Effects
Instruct patients to call their health care provider if they experience any signs or symptoms of dyspepsia or gastritis:
- Dyspepsia (upset stomach), burning, or nausea
- Abdominal pain or discomfort
- Epigastric discomfort, GERD (gastric indigestion)
17.4 Invasive or Surgical Procedures
Instruct patients to inform their health care provider that they are taking PRADAXA before any invasive procedure (including dental procedures) is scheduled.
17.5 Concomitant Medications
Ask patients to list all prescription medications, over-the-counter medications, or dietary supplements they are taking or plan to take so their health care provider knows about other treatments that may affect bleeding risk (e.g., aspirin or NSAIDs) or dabigatran exposure (e.g., dronedarone or systemic ketoconazole).
17.6 Prosthetic Heart Valves
Instruct patients to inform their health care provider if they will have or have had surgery to place a prosthetic heart valve.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield,
CT 06877 USA
Copyright 2014 Boehringer
Ingelheim Pharmaceuticals, Inc.
ALL RIGHTS RESERVED
75461-12
IT5060YD22014
75457-12
IT5400V
301841-07
IT5624J
MEDICATION GUIDE
PRADAXA (pra dax a)
(dabigatran etexilate mesylate)
capsules
Read this Medication Guide before you start taking PRADAXA and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important
information I should know about PRADAXA?
-
For people taking PRADAXA for atrial fibrillation:
People with atrial fibrillation (a type of irregular heartbeat) are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. PRADAXA lowers your chance of having a stroke by helping to prevent clots from forming. If you stop taking PRADAXA, you may have increased risk of forming a clot in your blood.
Do not stop taking PRADAXA without talking to the doctor who prescribes it for you. Stopping PRADAXA increases your risk of having a stroke.
PRADAXA may need to be stopped, if possible, prior to surgery or a medical or dental procedure. Ask the doctor who prescribed PRADAXA for you when you should stop taking it. Your doctor will tell you when you may start taking PRADAXA again after your surgery or procedure. If you have to stop taking PRADAXA, your doctor may prescribe another medicine to help prevent a blood clot from forming.
- PRADAXA can cause bleeding which can be serious, and sometimes
lead to death. This is because PRADAXA is a blood thinner medicine
that lowers the chance of blood clots forming in your body.
-
You may have a higher risk of bleeding if
you take PRADAXA and:
- are over 75 years old
- have kidney problems
- have stomach or intestine bleeding that is recent or keeps coming back, or you have a stomach ulcer
- take other medicines that increase your risk of bleeding,
including:
- aspirin or aspirin containing products
- long-term (chronic) use of non-steroidal anti-inflammatory drugs (NSAIDs)
- warfarin sodium (Coumadin®, Jantoven®)
- a medicine that contains heparin
- clopidogrel bisulfate (Plavix®)
- prasugrel (Effient®)
- have certain kidney problems and also take the medicines
dronedarone (Multaq®) or ketoconazole tablets
(Nizoral®).
Tell your doctor if you take any of these medicines. Ask your doctor or pharmacist if you are not sure if your medicine is one listed above.
- PRADAXA can increase your risk of bleeding because it lessens
the ability of your blood to clot. While you take PRADAXA:
- you may bruise more easily
- it may take longer for any bleeding to stop
Call your doctor or get medical help right away if you have any of these signs or symptoms of bleeding:- unexpected bleeding or bleeding that lasts a long time,
such as:
- unusual bleeding from the gums
- nose bleeds that happen often
- menstrual bleeding or vaginal bleeding that is heavier than normal
- bleeding that is severe or you cannot control
- pink or brown urine
- red or black stools (looks like tar)
- bruises that happen without a known cause or get larger
- cough up blood or blood clots
- vomit blood or your vomit looks like "coffee grounds"
- unexpected pain, swelling, or joint pain
- headaches, feeling dizzy or weak
Take PRADAXA exactly as prescribed. Do not stop taking PRADAXA without first talking to the doctor who prescribes it for you. Stopping PRADAXA may increase your risk of a stroke.
PRADAXA may need to be stopped, if possible, for one or more days before any surgery, or medical or dental procedure. If you need to stop taking PRADAXA for any reason , talk to the doctor who prescribed PRADAXA for you to find out when you should stop taking it. Your doctor will tell you when to start taking PRADAXA again after your surgery or procedure.
Spinal or epidural blood clots (hematoma). People who take a blood thinner medicine (anticoagulant) like PRADAXA, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:- a thin tube called an epidural catheter is placed in your back to give you certain medicine.
- you take NSAIDs or a medicine to prevent blood from clotting
- you have a history of difficult or repeated epidural or spinal punctures
- you have a history of problems with your spine or have had surgery on your spine.
If you take PRADAXA and receive spinal anesthesia or have a spinal puncture, your doctor should watch you closely for symptoms of spinal or epidural blood clots. Tell your doctor right away if you have back pain, tingling, numbness, muscle weakness (especially in your legs and feet), loss of control of the bowels or bladder (incontinence).
See "What are the possible side effects of PRADAXA?" for more information about side effects.
What is PRADAXA?
PRADAXA is a prescription blood thinner medicine that lowers the chance of blood clots forming in your body. PRADAXA is used to:
- reduce the risk of stroke and blood clots in people who have a medical condition called atrial fibrillation. With atrial fibrillation, part of the heart does not beat the way it should. This can lead to blood clots forming and increase your risk of a stroke.
- treat blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism) and reduce the risk of them occurring again.
PRADAXA is not for use in people with artificial (prosthetic) heart valves.
It is not known if PRADAXA is safe and works in children.
Who should not take PRADAXA?
Do not take PRADAXA if you:
- currently have certain types of abnormal bleeding. Talk to your doctor before taking PRADAXA if you currently have unusual bleeding.
- have had a serious allergic reaction to PRADAXA. Ask your doctor if you are not sure.
- have ever had or plan to have a valve in your heart replaced
What should I tell my doctor before taking PRADAXA?
Before you take PRADAXA, tell your doctor if you:
- have kidney problems
- have ever had bleeding problems
- have ever had stomach ulcers
- have any other medical condition
- are pregnant or plan to become pregnant. It is not known if PRADAXA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known
if PRADAXA passes into your breast milk.
Tell all of your doctors and dentists that you are taking PRADAXA. They should talk to the doctor who prescribed PRADAXA for you, before you have any surgery, or medical or dental procedure.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Some of your other medicines may affect the way PRADAXA works. Certain medicines may increase your risk of bleeding. See " What is the most important information I should know about PRADAXA ?"
Especially tell your doctor if you take:- rifampin (Rifater®, Rifamate®, Rimactane®, Rifadin®)
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take PRADAXA?
- Take PRADAXA exactly as prescribed by your doctor.
- Do not take PRADAXA more often than your doctor tells you to.
- You can take PRADAXA with or without food.
- You should take PRADAXA with a full glass of water.
- PRADAXA comes in a bottle or in a blister package.
- Only open 1 bottle of PRADAXA at a time. Finish your opened bottle of PRADAXA before opening a new bottle.
- After opening a bottle of PRADAXA, use within 4 months. See "How should I store PRADAXA?"
- When it is time for you to take a dose of PRADAXA, only remove your prescribed dose of PRADAXA from your open bottle or blister package.
- Tightly close your bottle of PRADAXA right away after you take your dose.
- Swallow PRADAXA capsules whole. Do not break, chew, or empty the pellets from the capsule.
- If you miss a dose of PRADAXA, take it as soon as you remember. If your next dose is less than 6 hours away, skip the missed dose. Do not take two doses of PRADAXA at the same time.
- Your doctor will decide how long you should take PRADAXA. Do not stop taking PRADAXA without first talking with your doctor. Stopping PRADAXA may increase your risk of stroke.
- Do not run out of PRADAXA. Refill your prescription before you run out. If you plan to have surgery, or a medical or a dental procedure, tell your doctor and dentist that you are taking PRADAXA. You may have to stop taking PRADAXA for a short time. See "What is the most important information I should know about PRADAXA?"
- If you take too much PRADAXA, go to the nearest hospital emergency room or call your doctor.
- Call your doctor or healthcare provider right away if you fall or injure yourself, especially if you hit your head. Your doctor or healthcare provider may need to check you.
What are the possible side effects of PRADAXA?
PRADAXA can cause serious side effects, including:
- See "What is the most important information I should know about PRADAXA?"
- Allergic Reactions. In some people, PRADAXA can cause symptoms
of an allergic reaction, including hives, rash, and itching. Tell
your doctor or get medical help right away if you get any of the following
symptoms of a serious allergic reaction with PRADAXA:
- chest pain or chest tightness
- swelling of your face or tongue
- trouble breathing or wheezing
- feeling dizzy or faint
Common side effects of PRADAXA include:
- indigestion, upset stomach, or burning
- stomach pain
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of PRADAXA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store PRADAXA?
- Store PRADAXA at room temperature between 59°F to 86°F (15°C to 30°C). After opening the bottle, use PRADAXA within 4 months. Safely throw away any unused PRADAXA after 4 months.
- Keep PRADAXA in the original bottle or blister package to keep it dry (protect the capsules from moisture). Do not put PRADAXA in pill boxes or pill organizers.
- Tightly close your bottle of PRADAXA right away after you take your dose.
Keep PRADAXA and all medicines out of the reach of children.
General information about PRADAXA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PRADAXA for a condition for which it was not prescribed. Do not give your PRADAXA to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about PRADAXA. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about PRADAXA that is written for health professionals.
For more information, go to www.PRADAXA.com or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257 or (TTY) 1-800-459-9906, or scan here to go to www.PRADAXA.com.

What are the ingredients in PRADAXA?
Active ingredient: dabigatran etexilate mesylate
Inactive ingredients: acacia, dimethicone, hypromellose, hydroxypropyl cellulose, talc, and tartaric acid. The capsule shell is composed of carrageenan, FD&C Blue No. 2 (150 mg strength only), FD&C Yellow No. 6, hypromellose, potassium chloride, titanium dioxide, and black edible ink.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Boehringer Ingelheim Pharmaceuticals,
Inc.
Ridgefield, CT 06877 USA
Revised: April 2014
The brands listed above are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc. The owners of these brands are not affiliated with and do not endorse Boehringer Ingelheim Pharmaceuticals, Inc., or its products.
Copyright 2014 Boehringer Ingelheim Pharmaceuticals,
Inc.
ALL RIGHTS RESERVED
75461-12
IT5060YD22014
75457-12
IT5400V
301841-07
IT5624J
PRADAXA (dabigatran etexilate mesylate)
75 mg capsules
NDC 0597-0149-54
PRADAXA (dabigatran etexilate mesylate)
75 mg capsules
NDC 0597-0149-60
PRADAXA (dabigatran etexilate mesylate)
75 mg capsules
NDC 0597-0149-54
PRADAXA (dabigatran etexilate mesylate)
150 mg capsules
NDC 0597-0135-54
PRADAXA (dabigatran etexilate mesylate)
150 mg capsules
NDC 0597-0135-60
PRADAXA (dabigatran etexilate mesylate)
150 mg capsules
NDC 0597-0135-12
PRADAXA (dabigatran etexilate mesylate)
150 mg capsules
NDC 0597-0135-54

Pradaxadabigatran etexilate mesylate CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pradaxadabigatran etexilate mesylate CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||