Pramipexole Dihydrochloride
Endo Pharmaceuticals Inc. DBA Endo Generic Products
Pramipexole Dihydrochloride Tablets 0.125 mg, 0.25 mg, 0.5 mg, 1 mg, and 1.5 mg Tablets Rx only Prescribing Information
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRAMIPEXOLE DIHYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- PRAMIPEXOLE DIHYDROCHLORIDE INDICATIONS AND USAGE
- PRAMIPEXOLE DIHYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE EVENTS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- PRAMIPEXOLE DIHYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- ANIMAL TOXICOLOGY
- PATIENT INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
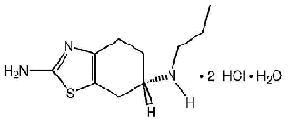
PRAMIPEXOLE DIHYDROCHLORIDE DESCRIPTION
S10173··2
CLINICAL PHARMACOLOGY
Mechanism of ActionParkinson’s Disease:
Pharmacokinetics
CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations
Absorption
max
Distribution
Metabolism and Elimination
Pharmacokinetics in Special Populations
CLINICAL PHARMACOLOGY, Renal Insufficiency
Gender
Age
CLINICAL PHARMACOLOGY, Renal Insufficiency
Parkinson's Disease Patients
Pediatric
Hepatic Insufficiency
Renal Insufficiency
PRECAUTIONS DOSAGE AND ADMINISTRATION
CLINICAL STUDIES
Parkinson's Disease
Studies in Patients with Early Parkinson's Disease
Studies in Patients with Advanced Parkinson's Disease
PRAMIPEXOLE DIHYDROCHLORIDE INDICATIONS AND USAGE
Parkinson's DiseasePRAMIPEXOLE DIHYDROCHLORIDE CONTRAINDICATIONS
Pramipexole dihydrochloride tablets are contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients.
WARNINGS
Falling Asleep During Activities of Daily LivingPatients treated with pramipexole dihydrochloride tablets have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles which sometimes resulted in accidents. Although many of these patients reported somnolence while on pramipexole dihydrochloride tablets, some perceived that they had no warning signs such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events had been reported as late as one year after the initiation of treatment.
Somnolence is a common occurrence in patients receiving pramipexole dihydrochloride tablets at doses above 1.5 mg/day (0.5 mg tid) for Parkinson’s disease. Many clinical experts believe that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, although patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with pramipexole dihydrochloride tablets, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with pramipexole dihydrochloride tablets such as concomitant sedating medications, the presence of sleep disorders, and concomitant medications that increase pramipexole plasma levels (e.g., cimetidine – see PRECAUTIONS, Drug Interactions). If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.), pramipexole dihydrochloride tablets should ordinarily be discontinued. If a decision is made to continue pramipexole dihydrochloride tablets, patients should be advised to not drive and to avoid other potentially dangerous activities. While dose reduction clearly reduces the degree of somnolence, there is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Symptomatic Hypotension
PRECAUTIONS
Hallucinations
PRECAUTIONS
RhabdomyolysisRenal
DOSAGE AND ADMINISTRATION
Dyskinesia
Retinal Pathology in Albino Rats
ANIMAL TOXICOLOGY
Events Reported with Dopaminergic Therapy
Withdrawal-Emergent Hyperpyrexia and Confusion
Fibrotic Complications
Melanoma
any
Information for Patients (also see Patient Package Insert)
PRECAUTIONSPregnancy
Laboratory Tests
During the development of pramipexole dihydrochloride tablets, no systematic abnormalities on routine laboratory testing were noted. Therefore, no specific guidance is offered regarding routine monitoring; the practitioner retains responsibility for determining how best to monitor the patient in his or her care.
Drug Interactions
Carbidopa/levodopa: maxmaxSelegiline:
Amantadine:
Cimetidine:
Probenecid:
Other drugs eliminated via renal secretion:
CYP interactions: in vivoin vitro
Dopamine antagonists:
Drug/Laboratory Test Interactions
There are no known interactions between pramipexole dihydrochloride tablets and laboratory tests.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
2in vitro in vivo
2
Pregnancy
Teratogenic Effect: Pregnancy Category C.
22Nursing Mothers
Pediatric Use
The safety and efficacy of pramipexole dihydrochloride tablets in pediatric patients has not been established.
Geriatric Use
Pramipexole total oral clearance was approximately 30% lower in subjects older than 65 years compared with younger subjects, because of a decline in pramipexole renal clearance due to an age-related reduction in renal function. This resulted in an increase in elimination half-life from approximately 8.5 hours to 12 hours. In clinical studies with Parkinson’s disease patients, 38.7% of patients were older than 65 years. There were no apparent differences in efficacy or safety between older and younger patients, except that the relative risk of hallucination associated with the use of pramipexole dihydrochloride tablets was increased in the elderly.
ADVERSE EVENTS
Parkinson's Disease
Early Parkinson's Disease
Adverse-event Incidence in Controlled Clinical Studies in Early Parkinson's Disease
|
Table 1 Treatment-Emergent Adverse-Event* Incidence in Double-Blind, Placebo-Controlled Trials in Early Parkinson's Disease (Events ≥1% of Patients Treated with Pramipexole Dihydrochloride Tablets and Numerically More Frequent Than in the Placebo Group) |
||
|
Body System/Adverse Event |
Pramipexole dihydrochloride |
Placebo |
|
|
N=388 |
N=235 |
|
Body as a Whole |
|
|
|
Asthenia |
14 |
12 |
|
General edema |
5 |
3 |
|
Malaise |
2 |
1 |
|
Reaction unevaluable |
2 |
1 |
|
Fever |
1 |
0 |
|
Digestive System |
|
|
|
Nausea |
28 |
18 |
|
Constipation |
14 |
6 |
|
Anorexia |
4 |
2 |
|
Dysphagia |
2 |
0 |
|
Metabolic & NutritionalSystem |
|
|
|
Peripheral edema |
5 |
4 |
|
Decreased weight |
2 |
0 |
|
Nervous System |
|
|
|
Dizziness |
25 |
24 |
|
Somnolence |
22 |
9 |
|
Insomnia |
17 |
12 |
|
Hallucinations |
9 |
3 |
|
Confusion |
4 |
1 |
|
Amnesia |
4 |
2 |
|
Hypesthesia |
3 |
1 |
|
Dystonia |
2 |
1 |
|
Akathisia |
2 |
0 |
|
Thinking abnormalities |
2 |
0 |
|
Decreased libido |
1 |
0 |
|
Myoclonus |
1 |
0 |
|
Special Senses |
|
|
|
Vision abnormalities |
3 |
0 |
|
Urogenital System |
|
|
|
Impotence |
2 |
1 |
Advanced Parkinson's Disease
Adverse-event Incidence in Controlled Clinical Studies in Advanced Parkinson's Disease
|
Table 2 Treatment-Emergent Adverse-Event* Incidence in Double-Blind, Placebo-Controlled Trials in Advanced Parkinson's Disease (Events ≥1% of Patients Treated with Pramipexole Dihydrochloride Tablets and Numerically More Frequent than in the Placebo Group) |
||
|
Body System/Adverse Event |
Pramipexole dihydrochloride† |
Placebo† |
|
|
N=260 |
N=264 |
|
Body as a Whole |
|
|
|
Accidental injury |
17 |
15 |
|
Asthenia |
10 |
8 |
|
General edema |
4 |
3 |
|
Chest pain |
3 |
2 |
|
Malaise |
3 |
2 |
|
Cardiovascular System |
|
|
|
Postural hypotension |
53 |
48 |
|
Digestive System |
|
|
|
Constipation |
10 |
9 |
|
Dry mouth |
7 |
3 |
|
Metabolic & Nutritional System |
|
|
|
Peripheral edema |
2 |
1 |
|
Increased creatine PK |
1 |
0 |
|
Musculoskeletal System |
|
|
|
Arthritis |
3 |
1 |
|
Twitching |
2 |
0 |
|
Bursitis |
2 |
0 |
|
Myasthenia |
1 |
0 |
|
Nervous System |
|
|
|
Dyskinesia |
47 |
31 |
|
Extrapyramidal syndrome |
28 |
26 |
|
Insomnia |
27 |
22 |
|
Dizziness |
26 |
25 |
|
Hallucinations |
17 |
4 |
|
Dream abnormalities |
11 |
10 |
|
Confusion |
10 |
7 |
|
Somnolence |
9 |
6 |
|
Dystonia |
8 |
7 |
|
Gait abnormalities |
7 |
5 |
|
Hypertonia |
7 |
6 |
|
Amnesia |
6 |
4 |
|
Akathisia |
3 |
2 |
|
Thinking abnormalities |
3 |
2 |
|
Paranoid reaction |
2 |
0 |
|
Delusions |
1 |
0 |
|
Sleep disorders |
1 |
0 |
|
Respiratory System |
|
|
|
Dyspnea |
4 |
3 |
|
Rhinitis |
3 |
1 |
|
Pneumonia |
2 |
0 |
|
Skin & Appendages |
|
|
|
Skin disorders |
2 |
1 |
|
Special Senses |
|
|
|
Accommodation |
|
|
|
Abnormalities |
4 |
2 |
|
Vision abnormalities |
3 |
1 |
|
Diplopia |
1 |
0 |
|
Urogenital System |
|
|
|
Urinary frequency |
6 |
3 |
|
Urinary tract infection |
4 |
3 |
|
Urinary incontinence |
2 |
1 |
General
Adverse Events; Relationship to Age, Gender, and Race
Other Adverse Events Observed During Phase 2 and 3 Clinical Trials
Blood and lymphatic system disorders:
Cardiac disorders:
Congenital, familial and genetic disorders
Ear and labyrinth disorders:
Endocrine disorders:
Eye disorders:
Gastrointestinal disorders:
General disorders:
Hepatobiliary disorders:
Immune system disorders:
Infections and infestations:
Injury, poisoning and procedural complications:
Metabolism and nutrition disorders:
Musculoskeletal and connective tissue disorders:
Neoplasms benign, malignant and unspecified:
Nervous system disorders:
Psychiatric disorders:
Renal and urinary disorders:
Reproductive system and breast disorders:
Respiratory, thoracic and mediastinal disorders:
Skin and subcutaneous tissue disorders:
Vascular disorders:
Falling Asleep During Activities of Daily Living
WARNING
Post-Marketing Experience
DRUG ABUSE AND DEPENDENCE
Pramipexole is not a controlled substance. Pramipexole has not been systematically studied in animals or humans for its potential for abuse, tolerance, or physical dependence. However, in a rat model on cocaine self-administration, pramipexole had little or no effect.
OVERDOSAGE
PRAMIPEXOLE DIHYDROCHLORIDE DOSAGE AND ADMINISTRATION
Parkinson's Disease
Dosing in Patients with Normal Renal Function
Initial Treatment
| Table 3 Ascending Dosage Schedule of Pramipexole Dihydrochloride Tablets for Parkinson's Disease | ||
| Week
|
Dosage (mg)
|
Total Daily Dose (mg)
|
| 1 |
0.125 TID |
0.375 |
| 2 |
0.25 TID |
0.75 |
| 3 |
0.5 TID |
1.50 |
| 4 |
0.75 TID |
2.25 |
| 5 |
1 TID |
3 |
| 6 |
1.25 TID |
3.75 |
| 7 |
1.5 TID |
4.50 |
Dosing in Patients with Renal Impairment
|
Table 4 Pramipexole Dosage in Parkinson's Disease Patients with Renal Impairment |
||
|
Renal Status |
Starting Dose (mg) |
Maximum Dose (mg) |
|
Normal to mild impairment (creatinine Cl > 60 mL/min) |
0.125 TID |
1.5 TID |
|
Moderate impairment (creatinine Cl =35 to 59 mL/min) |
0.125 BID |
1.5 BID |
|
Severe impairment (creatinine Cl =15 to 34 mL/min) |
0.125 QD |
1.5 QD |
|
Very severe impairment (creatinine Cl < 15 mL/min and hemodialysis patients) |
The use of pramipexole dihydrochloride has not been adequately studied in this group of patients |
|
Discontinuation of Treatment
HOW SUPPLIED
0.125 mg:
0.25 mg:
0.5 mg:
1 mg:
1.5 mg:
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F)
ANIMAL TOXICOLOGY
Retinal Pathology in Albino Rats
222
Fibro-osseous Proliferative Lesions in Mice
2
PATIENT INFORMATION
Pramipexole Dihydrochloride Tablets
Read the Patient Information that comes with pramipexole dihydrochloride before you start taking it and each time you get a refill. There may be some new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about pramipexole dihydrochloride?
Pramipexole dihydrochloride may cause you to fall asleep while you are doing daily activities such as driving, talking with other people, watching TV, or eating.
- Some people taking pramipexole dihydrochloride have had car accidents because they fell asleep while driving.
- Some patients did not feel sleepy before they fell asleep while driving. You could fall asleep without any warning.
Do not drive a car, operate a machine, or do anything that needs you to be alert until you know how pramipexole dihydrochloride affects you.
Tell your doctor right away if you fall asleep while you are doing activities such as talking with people, watching TV, eating, or driving, or if you feel sleepier than is normal for you.
What is pramipexole dihydrochloride?
Who should not take pramipexole dihydrochloride?
What should I tell my doctor before taking pramipexole dihydrochloride?
Tell your doctor about all of your medical conditions, including if you
- feel sleepy during the day from a sleep problem.
- have low blood pressure, or if you feel dizzy or faint, especially when getting up from a lying or sitting position.
- have trouble controlling your muscles (dyskinesia)
- have kidney problems.
- are pregnant or plan to become pregnant. It is not known if pramipexole dihydrochloride will harm your unborn baby.
- are breast feeding. It is not known if pramipexole dihydrochloride will pass into your breast milk. You and your doctor should decide if you will take – pramipexole dihydrochloride or breastfeed. You should not do both.
- drink alcohol. Alcohol can increase the chance that pramipexole dihydrochloride will make you feel sleepy or fall asleep when you should be awake.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take any other medicines that make you sleepy.
How should I take pramipexole dihydrochloride?
- Take pramipexole dihydrochloride exactly as your doctor tells you to. Your doctor will tell you how many pramipexole dihydrochloride Tablets to take and when to take them.
- Your doctor may change your dose until you are taking the right amount of medicine to control your symptoms. Do not take more or less pramipexole dihydrochloride than your doctor tells you to.
- Pramipexole dihydrochloride can be taken with or without food. Taking pramipexole dihydrochloride with food may lower your chances of getting nausea.
- If you miss a dose, do not double your next dose. Skip the dose you missed and take your next regular dose.
- Be sure to tell your doctor right away if you stop taking pramipexole dihydrochloride for any reason. Do not start taking pramipexole dihydrochloride again before speaking with your doctor. If you have Parkinson’s disease and are stopping pramipexole dihydrochloride, you should stop pramipexole dihydrochloride slowly over 7 days.
What should I avoid while taking pramipexole dihydrochloride?
- Do not drive a car, operate a machine, or do anything that needs you to be alert until you know how pramipexole dihydrochloride affects you. See “What is the most important information I should know about pramipexole dihydrochloride?” at the beginning of this leaflet.
- Do not drink alcohol while taking pramipexole dihydrochloride. It can increase your chances of feeling sleepy or falling asleep when you should be awake.
What are the possible side effects of pramipexole dihydrochloride?
Pramipexole dihydrochloride can cause serious side effects, including
- falling asleep during normal daily activities. See “What is the most important information I should know about pramipexole dihydrochloride?”
- low blood pressure when you sit or stand up quickly. You may have dizziness, nausea, fainting, or sweating. Sit and stand up slowly after you have been sitting or lying down for a while.
- hallucinations. You may see, hear, feel, or taste something that isn’t there. You have a higher chance of having hallucinations if you are over 65 years old.
Other Information about pramipexole dihydrochloride
How should I store pramipexole dihydrochloride?
- Store pramipexole dihydrochloride at room temperature at 59ºF to 86ºF (15ºC to 30ºC).
- Keep pramipexole dihydrochloride out of light.
- Keep pramipexole dihydrochloride and all medicines out of the reach of children.
General information about pramipexole dihydrochloride
What are the ingredients in pramipexole dihydrochloride?
Active Ingredient
Inactive Ingredients
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL





Pramipexole DihydrochloridePramipexole Dihydrochloride Tablets TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pramipexole DihydrochloridePramipexole Dihydrochloride Tablets TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pramipexole DihydrochloridePramipexole Dihydrochloride Tablets TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pramipexole DihydrochloridePramipexole Dihydrochloride Tablets TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pramipexole DihydrochloridePramipexole Dihydrochloride Tablets TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||