Prazolamine
HIGHLIGHTS OF PRESCRIBING INFORMATIONCarisoprodol Tablets, USP Rx only Rev. 09/09 These highlights do not include all the information needed to use Carisoprodol Tablets safely and effectively. See full prescribing information for Carisoprodol Tablets. Initial U.S. Approval Carisoprodol Tablets for Oral useRECENT MAJOR CHANGESIndications and Usage (1) 9/2007Dosage and Administration (2) 9/2007INDICATIONS AND USAGECarisoprodol Tablets are indicated for the relief of discomfort associatedwith acute, painful musculoskeletal conditions. (1) Important Limitations:• Should only be used for acute treatment periods up to two or threeweeks (1) • Not recommended in pediatric patients less than 16 years of age (8.4) DOSAGE AND ADMINISTRATION• Recommended dose is 350 mg three times a day and at bedtime. (2) DOSAGE FORMS AND STRENGTHSTablets: 350 mg (3) CONTRAINDICATIONS• Acute intermittent porphyria (4) • Hypersensitivity reactions to a carbamate such as meprobamate (4) WARNINGS AND PRECAUTIONS• Due to sedative properties, may impair ability to perform hazardoustasks such as driving or operating machinery (5.1) • Additive sedative effects when used with other CNS depressants includingalcohol (5.1) • Cases of Drug Dependence, Withdrawal, and Abuse (5.2) • Seizures (5.3) Side EffectsMost common adverse reactions (incidence > 2%) are drowsiness,dizziness, and headache (6.1) To report SUSPECTED ADVERSE REACTIONS, contact West-wardPharmaceutical Corp. at 1-877-233-2001 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.DRUG INTERACTIONS• CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclicantidepressants) – additive sedative effects ( 5.1 and 7.1)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 PRAZOLAMINE INDICATIONS AND USAGE
- 2 PRAZOLAMINE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 PRAZOLAMINE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS 5.1 Sedation 5.2 Drug Dependence, Withdrawal, and Abuse 5.3 Seizures
- 6 PRAZOLAMINE ADVERSE REACTIONS 6.1 Clinical Studies Experience 6.2 Post-marketing Experience
- 7 DRUG INTERACTIONS 7.1 CNS Depressants 7.2 CYP2C19 Inhibitors and Inducers
- 8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy: Pregnancy Category C.
- 8.2 Labor and Delivery
- 8.3 Nursing Mothers
- 8.4 Pediatric Use
- 8.5 Geriatric Use
- 8.6 Renal Impairment 8.7 Hepatic Impairment 8.8 Patients with Reduced CYP2C19 Activity
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 PRAZOLAMINE DESCRIPTION
- 12 CLINCIAL PHARMACOLOGY 12.1 Mechanism of Action
- 12.2 Pharmacodynamics
- 12.3 Pharmacokinetics
- 13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION 17.1 Sedation 17.2 Avoidance of Alcohol and Other CNS Depressants 17.3 Carisoprodol Should Only Be Used for Short-Term Treatment
FULL PRESCRIBING INFORMATION
Uses
1 INDICATIONS AND USAGE
1 INDICATIONS AND USAGE
Carisoprodol Tablets are indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults.
Carisoprodol Tablets should only be used for short periods (up to two or three weeks) because adequate evidence of effectiveness for more
prolonged use has not been established and because acute, painful musculoskeletal conditions are generally of short duration. [see Dosage and Administration (2)].
2 DOSAGE AND ADMINISTRATION
2 DOSAGE AND ADMINISTRATION
The recommended dose of Carisoprodol Tablets is 350 mg three times a day and at bedtime. The recommended maximum duration of Carisoprodol Tablets use is up to two or three weeks.
3 DOSAGE FORMS AND STRENGTHS
3 DOSAGE FORMS AND STRENGTHS
Carisoprodol Tablets, USP 350 mg are white, round, unscored tablets; imprinted “WW 176”.
4 CONTRAINDICATIONS
4 CONTRAINDICATIONS
Carisoprodol Tablets are contraindicated in patients with a history of acute intermittent porphyria or a hypersensitivity reaction to a carbamate such as meprobamate.
5 WARNINGS AND PRECAUTIONS 5.1 Sedation 5.2 Drug Dependence, Withdrawal, and Abuse 5.3 Seizures
5 WARNINGS AND PRECAUTIONS
5.1 Sedation
Carisoprodol Tablets may have sedative properties (in the low back pain trials, 13% to 17% of patients who received Carisoprodol Tablets experienced sedation compared to 6% of patients who received placebo) [see ADVERSE REACTIONS (6.1)] and may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a motor vehicle or operating machinery. Since the sedative effects of Carisoprodol Tablets and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive, appropriate caution should be exercised with patients who take more than one of these CNS depressants simultaneously.
5.2 Drug Dependence, Withdrawal, and Abuse In the postmarketing experience with Carisoprodol Tablets, cases of dependence, withdrawal, and abuse have been reported with prolonged use. Most cases of dependence withdrawal, and abuse occurred in patients
who have had a history of addiction or who used Carisoprodol Tablets in combination with other drugs with abuse potential. Withdrawal symptoms have been reported following abrupt cessation after prolonged use. To reduce the chance of Carisoprodol Tablets dependence, withdrawal, or abuse, Carisoprodol Tablets should be used with caution in addiction prone patients and in patients taking other CNS depressants including alcohol, and Carisoprodol Tablets should not be used more than two to three weeks for the relief of acute musculoskeletal discomfort. One of the metabolites of Carisoprodol Tablets, meprobamate (a controlled substance), may cause dependence. [see Clinical Pharmacology (12.3)].
5.3 Seizures
There have been postmarketing reports of seizures in patients who received Carisoprodol Tablets. Most of these cases have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol) [see Overdosage (10)].
6 ADVERSE REACTIONS 6.1 Clinical Studies Experience 6.2 Post-marketing Experience
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in practice. The data described below are based on 1387 patients pooled from two double blind, randomized, multicenter, placebo controlled, one-week trials in adult patients with acute, mechanical, lower back pain [see Clinical Studies (14)]. In the study, patients were treated with 350 mg of Carisoprodol Tablets, or placebo three times a day and at bedtime for seven days. The mean age was about 41 years old with 54% females and 46% males and 74% Caucasian, 16% Black, 9% Asian, and 2% other. There were no deaths and there were no serious adverse reactions in the trial. In the study, 2.7% and 5.4% of patients treated with placebo and 350 mg of Carisoprodol Tablets, respectively, discontinued due to adverse events; and 0.5% and 1.8% of patients treated with placebo and 350 mg of Carisoprodol Tablets, respectively, discontinued due to central nervous system adverse reactions.
Table 1 displays adverse reactions reported with frequencies greater than 2% and more frequently than placebo in patients treated with Carisoprodol Tablets in the trial described above.
| Adverse Reaction |
Placebo (n=560) n (%) |
Carisoprodol Tablets 350 mg (n=279) n (%) |
| Drowsiness |
31 (6) |
47 (17) |
| Dizziness |
11 (2) |
47 (17) |
| Headache |
11 (2) |
9 (3) |
7 DRUG INTERACTIONS 7.1 CNS Depressants 7.2 CYP2C19 Inhibitors and Inducers
7 DRUG INTERACTIONS
7.1 CNS Depressants
The sedative effects of Carisoprodol Tablets and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously. Concomitant use of Carisoprodol Tablets and meprobamate, a metabolite of Carisoprodol Tablets, is not recommended [see Warnings and Precautions (5.1)].
7.2 CYP2C19 Inhibitors and Inducers Carisoprodol Tablets are metabolized in the liver by CYP2C19 to form meprobamate [see Clinical Pharmacology (12.3)]. Co-administration of CYP2C19 inhibitors, such as omeprazole or fluvoxamine, with Carisoprodol Tablets could result in increased exposure of carisoprodol and decreased exposure of meprobamate. Co-administration of CYP2C19 inducers,such as rifampin or St. John’s Wort, with Carisoprodol Tablets could result in decreased exposure of carisoprodol and increased exposure of meprobamate. Low dose aspirin also showed induction effect on CYP2C19.
The full pharmacological impact of these potential alterations of exposures in terms of either efficacy or safety of Carisoprodol Tablets is unknown.
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy: Pregnancy Category C.
8.1 Pregnancy: Category Pregnancy C.
There are no data on the use of Carisoprodol Tablets during human pregnancy. Animal studies indicate that carisoprodol crosses the placenta and results in adverse effects on fetal growth and postnatal survival. The primary metabolite of carisoprodol, meprobamate, is an approved anxiolytic. Retrospective, post-marketing studies do not show a consistent association between maternal use of meprobamate and an increased risk for particular congenital malformations. Teratogenic effects: Animal studies have not adequately evaluated the teratogenic effects of carisoprodol. There was no increase in the incidence of congenital malformations noted in reproductive studies in rats, rabbits, and mice treated with meprobamate. Retrospective, post-marketing studies of meprobamate during human pregnancy were equivocal for demonstrating an increased risk of congenital malformations following first trimester exposure. Across studies that indicated an increased risk, the types of malformations were inconsistent. Nonteratogenic effects: In animal studies, carisoprodol reduced fetal weights, postnatal weight gain, and postnatal survival at maternal doses equivalent to 1-1.5 times the human dose (based on a body surface area comparison). Rats exposed to meprobamate in utero showed behavioral alterations that persisted into adulthood. For children exposed to meprobamate in utero, one study found no adverse effects on mental or motor development or IQ scores. Carisoprodol Tablets should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
8.2 Labor and Delivery
8.2 Labor and Delivery
There is no information about the effects of Carisoprodol Tablets on the mother and the fetus during labor and delivery.
8.3 Nursing Mothers
8.3 Nursing Mothers
Very limited data in humans show that Carisoprodol Tablets is present in breast milk and may reach concentrations two to four times the maternal plasma concentrations. In one case report, a breast-fed infant received about 4-6% of the maternal daily dose through breast milk and experienced no adverse effects. However, milk production was inadequate and the baby was supplemented with formula. In lactation studies in mice, female pup survival and pup weight at weaning were decreased. This information suggests that maternal use of Carisoprodol Tablets may lead to reduced or less effective infant feeding (due to sedation) and/or decreased milk production. Caution should be exercised when Carisoprodol Tablets are administered to a nursing woman
8.4 Pediatric Use
8.4 Pediatric Use
The efficacy, safety, and pharmacokinetics of Carisoprodol Tablets in pediatric patients less than 16 years of age have not been established.
8.5 Geriatric Use
8.5 Geriatric Use
The efficacy, safety, and pharmacokinetics of Carisoprodol Tablets in patients over 65 years old have not been established.
8.6 Renal Impairment 8.7 Hepatic Impairment 8.8 Patients with Reduced CYP2C19 Activity
8.6 Renal Impairment
The safety and pharmacokinetics of Carisoprodol Tablets in patients with renal impairment have not been evaluated. Since Carisoprodol Tablets are excreted by the kidney, caution should be exercised if Carisoprodol Tablets are administered to patients with impaired renal function. Carisoprodol Tablets are dialyzable by hemodialysis and peritoneal dialysis.
8.7 Hepatic Impairment
The safety and pharmacokinetics of Carisoprodol Tablets in patients with hepatic impairment have not been evaluated. Since Carisoprodol Tablets are metabolized in the liver, caution should be exercised if Carisoprodol Tablets are administered to patients with impaired hepatic function.
8.8 Patients with Reduced CYP2C19 Activity
Patients with reduced CYP2C19 activity have higher exposure to Carisoprodol Tablets. Therefore, caution should be exercised in administration of Carisoprodol Tablets to these patients. [see Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9 DRUG ABUSE AND DEPENDENCE
[see Warnings and Precautions (5.2)]
5.2 Drug Dependence, Withdrawal, and Abuse
In the postmarketing experience with Carisoprodol Tablets, cases of dependence, withdrawal, and abuse have been reported with prolonged use. Most cases of dependence withdrawal, and abuse occurred in patients who have had a history of addiction or who used Carisoprodol Tablets in combination with other drugs with abuse potential. Withdrawal symptoms have been reported following abrupt cessation after prolonged use. To reduce the chance of Carisoprodol Tablets dependence, withdrawal, or abuse, Carisoprodol Tablets should be used with caution in addictionprone patients and in patients taking other CNS depressants including alcohol, and Carisoprodol Tablets should not be used more than two to three weeks for the relief of acute musculoskeletal discomfort.
One of the metabolites of Carisoprodol Tablets, meprobamate (a controlled substance), may cause dependence. [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
10 OVERDOSAGE
Overdosage of Carisoprodol Tablets commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic reactions, nystagmus, blurred vision, mydriasis, euphoria, muscular incoordination, rigidity, and/or headache have been reported with Carisoprodol Tablets overdosage. Many of the Carisoprodol Tablets over-doses have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol). The effects of an overdose of Carisoprodol Tablets and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) can be additive even when one of the drugs has been taken in the recommended dosage. Fatal accidental and non-accidental overdoses of Carisoprodol Tablets have been reported alone or in combination with CNS depressants.
Treatment of Overdosage: Basic life support measures should be instituted as dictated by the clinical presentation of Carisoprodol Tablets overdose. Induced emesis is not recommended due to the risk of CNS and respiratory depression, which may increase the risk of aspiration pneumonia. Gastric lavage should be considered soon after ingestion (within one hour). Circulatory support should be administered with volume infusion and vasopressor agents if needed. Seizures should be treated with intravenous benzodiazepines and the reoccurrence of seizures may be treated with phenobarbital. In cases of severe CNS depression, airway protective reflexes may be compromised and tracheal intubation should be considered for airway protection and respiratory support. The following types of treatment have been used successfully with an overdose of meprobamate, a metabolite of carisoprodol: activated charcoal (oral or via nasogastric tube), forced diuresis, peritoneal dialysis, and hemodialysis (carisoprodol is also dialyzable). Careful monitoring of urinary output is necessary and overhydration should be avoided. Observe for possible relapse due to incomplete gastric emptying and delayed absorption. For more information on the management of an overdose of Carisoprodol Tablets, contact a Poison Control Center.
11 DESCRIPTION
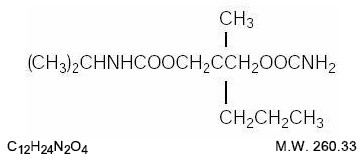 11 DESCRIPTION
11 DESCRIPTION
Carisoprodol Tablets, USP are available as 350 mg round, white tablets. Carisoprodol is a white, crystalline powder, having a mild, characteristic odor and a bitter taste. It is slightly soluble in water; freely soluble in alcohol, in chloroform, and in acetone; and its solubility is practically independent of pH. Carisoprodol is present as a racemic mixture. Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3- propanediol dicarbamate and the molecular formula is C12H24N2O4 , with a molecular weight of 260.33. The structural formula is:
Other Ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, methylcellulose, povidone, sodium lauryl sulphate, sodium starch glycolate, and stearic acid.
12 CLINCIAL PHARMACOLOGY 12.1 Mechanism of Action
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified.
In animal studies, muscle relaxation induced by carisoprodol is associated with altered interneuronal activity in the spinal cord and in the descending reticular formation of the brain.
12.2 Pharmacodynamics
12.2 Pharmacodynamics
Carisoprodol is a centrally acting skeletal muscle relaxant that does not directly relax skeletal muscles. A metabolite of carisoprodol, meprobamate, has anxiolytic and sedative properties. The degree to which these properties of meprobamate contribute to the safety and efficacy of Carisoprodol Tablets is unkown.
12.3 Pharmacokinetics
12.3 Pharmacokinetics
The pharmacokinetics of carisoprodol and its metabolite meprobamate were studied in a crossover study of 24 healthy subjects (12 male and 12 female) who received single doses 350 mg carisoprodol (see Table 2). The Cmax of meprobamate was 2.5 ± 0.5 μg/mL (mean ± SD) after administration of a single 350 mg dose of carisoprodol, which is approximately 30% of the Cmax of meprobamate (approximately 8 μg/mL) after administration of a single 400 mg dose of meprobamate.
|
|
Carisoprodol 350 mg |
| Carisoprodol |
Carisoprodol |
| Cmax (μg/mL) |
1.8 ± 1.0 |
| AUCinf (μg*hr/mL) |
7.0 ± 5.0 |
| Tmax (hr) |
1.7 ± 0.8 |
| T1/2 (hr) |
2.0 ± 0.5 |
| Meprobamate |
Meprobamate |
| Cmax (μg/mL) |
2.5 ± 0.5 |
| AUCinf (μg*hr/mL) |
46 ± 9.0 |
| Tmax (hr) |
4.5 ± 1.9 |
| T1/2 (hr) |
9.6 ± 1.5 |
Absorption: Absolute bioavailability of carisoprodol has not been determined. The mean time to peak plasma concentrations (Tmax) of carisoprodol was approximately 1.5 to 2 hours. Co-administration of a high-fat meal with carisoprodol (350 mg tablet) had no effect on the pharmacokinetics of carisoprodol. Therefore, Carisoprodol Tablets may be administered with or without food.
Metabolism: The major pathway of carisoprodol metabolism is via the liver by cytochrome enzyme CYP2C19 to form meprobamate. This enzyme exhibits genetic polymorphism (see Patients with Reduced CYP2C19 Activity below).
Elimination: Carisoprodol is eliminated by both renal and non-renal routes with a terminal elimination half-life of approximately 2 hours. The half-life of meprobamate is approximately 10 hours.
Gender: Exposure of carisoprodol is higher in female than in male subjects (approximately 30-50% on a weight adjusted basis). Overall exposure of meprobamate is comparable between female and male subjects. Patients with Reduced CYP2C19 Activity: Carisoprodol Tablets should be used with caution in patients with reduced CYP2C19 activity. Published studies indicate that patients who are poor CYP2C19 metabolizers have a 4-fold increase in exposure to carisoprodol, and concomitant 50% reduced exposure to meprobamate compared to normal CYP2C19 metabolizers. The prevalence of poor metabolizers in Caucasians and African Americans is approximately 3-5% and in Asians is approximately 15-20%.
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed to evaluate the carcinogenic potential of carisoprodol. Carisoprodol Tablets were not formally evaluated for genotoxicity. In published studies, carisoprodol was mutagenic in the in vitro mouse lymphoma cell assay in the absence of metabolizing enzymes, but was not mutagenic in the presence of metabolizing enzymes. Carisoprodol was clastogenic in the in vitro chromosomal aberration assay using Chinese hamster ovary cells with or without the presence of metabolizing enzymes.
Other types of genotoxic tests resulted in negative findings. Carisoprodol was not mutagenic in the Ames reverse mutation assay using S. typhimurium strains with or without metabolizing enzymes, and was not clastogenic in an in vivo mouse micronucleus assay of circulating blood cells. Carisoprodol Tablets were not formally evaluated for effects on fertility. Published reproductive studies of carisoprodol in mice found no alteration in fertility although an alteration in reproductive cycles characterized by a greater time spent in estrus was observed at a carisoprodol dose of 1200 mg/kg/day. In a 13-week toxicology study that did not determine fertility, mouse testes weight and sperm motility were reduced at a dose of 1200 mg/kg/day. In both studies, the no effect level was 750 mg/kg/day, corresponding to approximately 2.6 times the human equivalent dosage of 350 mg four times a day, based on a body surface area comparison. The significance of these findings for human fertility is not known.
14 CLINICAL STUDIES
14 CLINICAL STUDIES
The safety and efficacy of Carisoprodol Tablets for the relief of acute, idiopathic mechanical low back pain was evaluated in one, 7-day, double blind, randomized, multicenter, placebo controlled, U.S. trial (Study 1). Patients had to be 18 to 65 years old and had to have acute back pain (≤ 3 days of duration) to be included in the trials. Patients with chronic back pain; at increased risk for vertebral fracture (e.g., history of osteoporosis); with a history of spinal pathology (e.g., herniated nucleus pulposis, spondylolisthesis or spinal stenosis); with inflammatory back pain, or with evidence of a neurologic deficit were excluded from participation. Concomitant use of analgesics (e.g., acetaminophen, NSAIDs, tramadol, opioid agonists), other muscle relaxants, botulinum toxin, sedatives (e.g., barbiturates, benzodiazepines, promethazine hydrochloride), and anti-epileptic drugs was prohibited.
In Study 1, patients were randomized to one of two treatment groups (i.e., carisoprodol 350 mg or placebo). In the study, patients received study medication three times a day and at bedtime for seven days. The primary endpoints were the relief from starting backache and the global impression of change, as reported by patients, on Study Day #3. Both endpoints were scored on a 5-point rating scale from 0 (worst outcome) to 4 (best outcome) in the study. The proportion of patients who used concomitant acetaminophen, NSAIDs,
tramadol, opioid agonists, other muscle relaxants, and benzodiazepines was similar in the treatment groups. The results for the primary efficacy evaluations in the acute, low back pain study are presented in Table 3.
| Study |
Parameter |
Placebo |
Carisoprodol Tablets 350 mg |
| 1 |
Number of Patients |
n=269 |
n=273 |
| 1 |
Relief from Starting Backache, Mean (SE)b |
1.4(0.1) |
1.8(0.1) |
| 1 |
Difference between Carisoprodol Tablets and Placebo, Mean (SE)b (95% Cl) |
|
0.4 (0.2, 0.6) |
| 1 |
Global Impression of Change, Mean (SE)b |
1.9(0.1) |
2.2(0.1) |
| 1 |
Difference between Carisoprodol Tablets and Placebo, Mean (SE)b (95% Cl) |
|
0.3 (0.1, 0.4) |
16 HOW SUPPLIED/STORAGE AND HANDLING
16 HOW SUPPLIED/STORAGE AND HANDLING
Carisoprodol Tablets, USP 350 mg, are white, round, unscored tablets; imprinted “WW 176”, are available in:
Bottles of 100 tablets
Bottles of 500 tablets
Bottles of 1000 tablets
Storage:
Store at 20°-25°C (68°-77°F). [See USP Controlled Room Temperature.] Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
17 PATIENT COUNSELING INFORMATION 17.1 Sedation 17.2 Avoidance of Alcohol and Other CNS Depressants 17.3 Carisoprodol Should Only Be Used for Short-Term Treatment
17 PATIENT COUNSELING INFORMATION
Patients should be advised to contact their physician if they experience any adverse reactions to Carisoprodol Tablets.
17.1 Sedation
Since Carisoprodol Tablets may cause drowsiness and/or dizziness, patients should be advised to assess their individual response to Carisoprodol Tablets before engaging in potentially hazardous activities such as driving a motor vehicle or operating machinery [see Warnings and Precautions (5.1)].
17.2 Avoidance of Alcohol and Other CNS Depressants
Patients should be advised to avoid alcoholic beverages while taking Carisoprodol Tablets and to check with their doctor before taking other CNS depressants such as benzodiazepines, opioids, tricyclic antidepressants, sedating antihistamines, or other sedatives [see Warnings and Precautions (5.1)].
17.3 Carisoprodol Tablets Should Only Be Used for Short-Term Treatment
Patients should be advised that treatment with Carisoprodol Tablets should be limited to acute use (up to two or three weeks) for the relief of acute, musculoskeletal discomfort. If symptoms still persist, patients should contact their healthcare provider for further evaluation.
Manufactured for: West-ward Pharmaceutical Corp Eatontown, NJ 07724
Manufactured by: Shasun Chemical and Drugs Ltd. Pondicherry, India
Carisoprodol 350mg
Packaged by Bryant Ranch North Hollywood CA 91605
Carisprodol 350 mg (CIV) Tablet
Compare To
Soma 350mg (CIV) Tablet
WEST WARD PHARMACEUTICAL CORP
#60 EXP 03/11
NDC 6362913083
LOT 10038
May Cause Drowsiness/No Alcohol
Keep all drugs out of reach of children
PRODUCT DESCRIPTION
Primary Ingredients Theramine consists of a proprietary blend of amino acids, cocoa, caffeine, cinnamon, and flavonoids in specific proportions. These ingredients fall into the category of Generally Regarded as Safe” (GRAS) as defined by the Food and Drug Administration (FDA) (Sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act). A GRAS substance is distinguished from a food additive on the basis of the common knowledge about the safety of the substance for its intended use. The standard for an ingredient to achieve GRAS status requires not only technical demonstration of non-toxicity and safety, but also general recognition of safety through widespread usage and agreement of that safety by experts in the field. Many ingredients have been determined by the U.S. Food and Drug Administration (FDA) to be GRAS, and are listed as such by regulation, in Volume 21 Code of Federal Regulations (CFR) Sections 182, 184, and 186.
Amino Acids
Amino Acids are the building blocks of protein. All amino acids are GRAS listed as they have been ingested by humans for thousands of years. The doses of the amino acids in Theramine are equivalent to those found in the usual human diet. Patients with pain disorders may require an increased amount of certain amino acids that cannot be obtained from normal diet alone. Tryptophan, for example, is an obligatory amino acid. The body cannot make tryptophan and must obtain tryptophan from the diet. Tryptophan is needed to produce serotonin. Serotonin is required to reduce pain. Patients with pain disorders and inflammatory conditions have altered serotonin metabolism. Some patients with pain disorders and inflammatory conditions have a resistance to the use of tryptophan that is similar to the mechanism found in insulin resistance. Patients with pain disorders and inflammatory conditions cannot acquire sufficient tryptophan from the diet to alter the perception of pain and the inflammatory process without ingesting a prohibitively large amount of calories, particularly calories from protein.
Flavonoids
Flavonoids are a group of phytochemical compounds found in all vascular plants including fruits and vegetables. They are a part of a larger class of compounds known as polyphenols. Many of the therapeutic or health benefits of colored fruits and vegetables, cocoa, red wine, and green tea are directly related to their flavonoid content. The specially formulated flavonoids found in Theramine cannot be obtained from conventional foods in the necessary proportions to elicit a therapeutic response.
Other Ingredients
Theramine contains the following inactive or other ingredients, as fillers, excipients, and colorings: magnesium stearate, microcrystalline cellulose, Maltodextrin NF, gelatin (as the capsule material).
Physical Description
Theramine is a yellow to light brown powder. Theramine contains L-Glutamine, L-Arginine, L-Histidine, and L-Serine, 5-Hydroxytryptophan as Griffonia Seed Extract, GABA, Choline Bitartrate, Cinnamon, Cocoa, Hydrolyzed Whey Protein, and Grape Seed Extract.
CLINICAL PHARMACOLOGY
Mechanism of Action
Theramine acts by restoring and maintaining the balance of the neurotransmitters; GABA, nitric oxide, serotonin, and acetylcholine that are associated with pain disorders and inflammatory conditions. Theramine stimulates the production ACTH to reduce inflammation.
Metabolism
The amino acids in Theramine are primarily absorbed by the stomach and small intestines. All cells metabolize the amino acids in Theramine. Circulating tryptophan, arginine and choline blood levels determine the production of serotonin, nitric oxide, and acetylcholine.
Excretion
Theramine is not an inhibitor of cytochrome P450 1A2, 2C9, 2C19, 2D6, or 3A4. These isoenzymes are principally responsible for 95% of all detoxification of drugs, with CYP3A4 being responsible for detoxification of roughly 50% of drugs. Amino acids do not appear to have an effect on drug metabolizing enzymes.
Uses
INDICATIONS FOR USE
Theramine is intended for the clinical dietary management of the metabolic processes of pain disorders and inflammatory conditions.
CLINICAL EXPERIENCE
Administration of Theramine has demonstrated significant reduction in symptoms of pain and inflammation in patients with acute and chronic pain when used for the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Administration of Theramine results in the induction and maintenance of pain relief in patients with pain disorders and inflammatory conditions.
PRECAUTIONS AND CONTRAINDICATIONS
Theramine is contraindicated in an extremely small number of patients with hypersensitivity to any of the nutritional components of Theramine.
ADVERSE REACTIONS
Oral supplementation with L-tryptophan, L-arginine or choline at high doses up to 15 grams daily is generally well tolerated. The most common adverse reactions of higher doses — from 15 to 30 grams daily — are nausea, abdominal cramps, and diarrhea. Some patients may experience these symptoms at lower doses. The total combined amount of amino acids in each Theramine capsule does not exceed 400 mg
DRUG INTERACTIONS
Theramine does not directly influence the pharmacokinetics of prescription drugs. Clinical experience has shown that administration of Theramine may allow for lowering the dose of co-administered drugs under physician supervision.
OVERDOSE
There is a negligible risk of overdose with Theramine as the total dosage of amino acids in a one month supply (90 capsules) is less than 36 grams. Overdose symptoms may include diarrhea, weakness, and nausea.
POST-MARKETING SURVEILLANCE
Post-marketing surveillance has shown no serious adverse reactions. Reported cases of mild rash and itching may have been associated with allergies to Theramine flavonoid ingredients, including cinnamon, cocoa, and chocolate. These reactions were transient in nature and subsided within 24 hours.
DOSAGE AND ADMINISTRATION
Recommended Administration For the dietary management of the metabolic processes associated with pain disorders and inflammatory conditions. Take (2) capsules one to three times daily or as directed by physician. As with most amino acid formulations Theramine should be taken without food to increase the absorption of key ingredients.
How Supplied
Theramine is supplied in purple and white, size 0 capsules in bottles of 60 or 90 capsules.
Physician Supervision
Theramine is a Medical Food product available by prescription only and must be used while the patient is under ongoing physician supervision.
U.S. patent pending.
Manufactured by Arizona Nutritional Supplements, Inc. Chandler AZ 85225
Distributed by Physician Therapeutics LLC, Los Angeles, CA 90077. www.ptlcentral.com
Copyright 2003-2006, Physician Therapeutics LLC, all rights reserved
NDC: 68405-1008-02
NDC: 68405-1008-03
Storage
Store at room temperature, 59-86OF (15-30OC) Protect from light and moisture. Theramine is supplied to physicians in a recyclable plastic bottle with a child-resistant cap.
PHYSICIAN THERAPEUTICS THERAMINE Medical Food Rx only 90 Capsules Directions for use: Must be administered under medical supervision. For adults only. As a Medical Food, take one (1) or two (2) capsules every four hours or as directed by your medical practitioner. For the dietary management of Myalgia. Contains no added sugar, starch, wheat, yeast, preservatives, artificial flavor. Storage: Keep tightly closed in a cool dry place 8-320 C (45-900 F), relative humidity, below 50%. Warning: Keep this product out of the reach of children. NDC# 68405-1008-03 Ingredients: Each serving (per 2 capsules) contains: Proprietary Amino Acid Blend Griffonia Seed Extract (5-HTP), Whey Protein Hydrolysate, L-Arginini, L-Histidine (as L-Histidine HCl), L-Glutamine, L-Serine, Gamma Amino Butyric Acid, Choline Bitartrate, Cocoa (6% Theobromine) (fruit), Grape Extract (95% Polyphenols) (seed), Cinnamon (bark) Other Ingredients: Gelatin, Silicon Dioxide, Tricalcium phosphate, Vegetable Magnesium Stearate, Cellulose, FD and C Blue #1, FD and C red#3, titanium dioxide. Distributed by: Physician Therapeutics LLC, Los Angeles, CA 90077 www.ptlcentral.com Patent Pending
A convenience Packed Medical Food and Drug
Prazolamine
Physician Therapeutics
Theramine 60 Capsules
Carisoprodol 350mg 30 Tablets
No Refills Without Physician Authorization
Rx Only
NDC # 68405-8028-06 of this co-pack
For the Dietary Management of Pain and Inflammation.
Two capsules twice daily or as directed byphysician. See product label and insert
Theramine
Medical Food
As prescribed by physician. See product label and product information insert.
Carisoprodol 350mg
Rx Drug
Physician Therapeutics LLC
Los Angeles, CA 90077
on November 21, 2006


PrazolamineCARISOPRODOL, GABA KIT
| ||||||||||||||||||||||||||||||||||||||||