Prednisone
FULL PRESCRIBING INFORMATION: CONTENTS*
- PREDNISONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PREDNISONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PREDNISONE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PREDNISONE DESCRIPTION
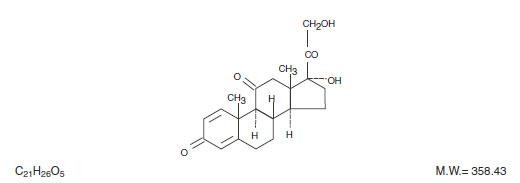
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
PREDNISONE CONTRAINDICATIONS
WARNINGS
GeneralADVERSE REACTIONS: Allergic Reactions).
Cardio-Renal
Endocrine
Infection
PRECAUTIONS: Drug Interactions: Amphotericin B Injection and Potassium-Depleting Agents).
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered. However, the response to such vaccines may be diminished and cannot be predicted.Indicated immunization procedures may be undertaken in patients receiving nonimmunosuppressive doses of corticosteroids as replacement therapy (e.g., for Addisondisease).
PRECAUTIONS
General PrecautionsCardio-Renal
Endocrine
Gastrointestinal
Musculoskeletal
Neuro-Psychiatric
DOSAGE AND ADMINISTRATION: Multiple Sclerosis.)
Ophthalmic
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
amphotericin B, diuretics), patients should be observed closely for development of hypokalemia. In addition, there have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure.
PRECAUTIONS: Drug Interactions: Hepatic Enzyme Inducers, Inhibitors and Substrates).
neostigmine, pyridostigmine) and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy. If concomitant therapy must occur, it should take place under close supervision and the need for respiratory support should be anticipated.
warfarinusually results in inhibition of response to warfarin, although there have been some conflicting reports. Therefore, coagulation indices should be monitored frequently to maintain the desired anticoagulant effect.
isoniazidmay be decreased.
ciprofloxacin, levofloxacin) and corticosteroids, especially in the elderly. Tendon rupture can occur during or after treatment with quinolones.
inducecytochrome P450 3A4 (CYP 3A4) enzyme activity (e.g., barbiturates, phenytoin, carbamazepine, rifampin) may enhance the metabolism of corticosteroids and require that the dosage of the corticosteroid be increased. Drugs which inhibitCYP 3A4 (e.g., ketoconazole, itraconazole, ritonavir, indinavir, macrolide antibiotics such as erythromycin) have the potential to result in increased plasma concentrations of corticosteroids. Glucocorticoids are moderate inducers of CYP 3A4. Co-administration with other drugs that are metabolized by CYP 3A4 (e.g., indinavir, erythromycin) may increase their clearance, resulting in decreased plasma concentration.
aspirin(or other nonsteroidal anti-inflammatory agents
WARNINGS: Infection: Vaccination).
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Pediatric patients who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression (i.e., cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The linear growth of pediatric patients treated with corticosteroids should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of treatment alternatives. In order to minimize the potential growth effects of corticosteroids, pediatric patients should be titrated to the lowest effective dose.
GERIATRIC USE
PREDNISONE ADVERSE REACTIONS
listed alphabetically, under each subsection)The following adverse reactions have been reported with prednisone or other corticosteroids:
Allergic Reactions
anaphylactoid or hypersensitivity reactions, anaphylaxis, angioedema.
WARNINGS: Cardio-Renal), necrotizing angiitis, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis.
PRECAUTIONS: General Precautions), lupus erythematosus-like lesions, perineal irritation, purpura, rash, striae, subcutaneous fat atrophy, suppression of reactions to skin tests, striae, telangiectasis, thin fragile skin, thinning scalp hair, urticaria.
WARNINGS: Endocrine), hypothyroidism, increased requirements for insulin or oral hypoglycemic agents in diabetics, lipids abnormal, moon face, negative nitrogen balance caused by protein catabolism, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery or illness) (see WARNINGS: Endocrine), suppression of growth in pediatric patients.
PRECAUTIONS: Musculoskeletal), pathologic fracture of long bones, steroid myopathy, tendon rupture (particularly of the Achilles tendon), vertebral compression fractures.
PRECAUTIONS: Ophthalmic), optic nerve damage, papilledema.
WARNINGS: Infection), hiccups, immunosuppression, increased or decreased motility and number of spermatozoa, malaise, insomnia, moon face, pyrexia.
DOSAGE & ADMINISTRATION
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.
Multiple Sclerosis
Alternate Day Therapy
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PrednisonePREDNISONE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!