PREDNISONE
FULL PRESCRIBING INFORMATION: CONTENTS*
- PREDNISONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PREDNISONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PREDNISONE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PREDNISONE DESCRIPTION
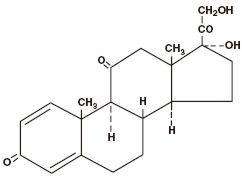
1 mg: anhydrous lactose, corn starch, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate.|
2.5 mg:anhydrous lactose, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and talc.
5 mg: anhydrous lactose, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and talc.
10 mg:anhydrous lactose, colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and talc.
20 mg: anhydrous lactose, D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
� Endocrine disorders
Congenital adrenal hyperplasia
Nonsuppurative thyroiditis
Hypercalcemia associated with cancer
� Rheumatic disorders
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Psoriatic arthritis
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy)
Ankylosing spondylitis
Acute and subacute bursitis
Acute nonspecific tenosynovitis
Acute gouty arthritis
Post-traumatic osteoarthritis
Synovitis of osteoarthritis
Epicondylitis
� Collagen diseases
During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus
Systemic dermatomyositis (polymyositis)
Acute rheumatic carditis
� Dermatologic diseases
Pemphigus
Bullous dermatitis herpetiformis
Severe erythema multiforme (Stevens-Johnson syndrome)
Exfoliative dermatitis
Mycosis fungoides
Severe psoriasis
Severe seborrheic dermatitis
� Allergic states
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment:
Seasonal or perennial allergic rhinitis
Serum sickness
Bronchial asthma
Contact dermatitis
Atopic dermatitis
Drug hypersensitivity reactions
� Ophthalmic diseases
Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as:
Allergic conjunctivitis
Keratitis
Allergic corneal marginal ulcers
Herpes zoster ophthalmicus
Iritis and iridocyclitis
Chorioretinitis
Anterior segment inflammation
Diffuse posterior uveitis and choroiditis
Optic neuritis
Sympathetic ophthalmia
� Respiratory diseases
Symptomatic sarcoidosis
Loeffler's syndrome not manageable by other means
Berylliosis
Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy
Aspiration pneumonitis
� Hematologic disorders
Idiopathic thrombocytopenic purpura in adults
Secondary thrombocytopenia in adults
Acquired (autoimmune) hemolytic anemia
Erythroblastopenia (RBC anemia)
Congenital (erythroid) hypoplastic anemia
� Neoplastic diseases
For palliative management of:
Leukemias and lymphomas in adults
Acute leukemia of childhood
� Edematous states
To induce a diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus
� Gastrointestinal diseases
To tide the patient over a critical period of the disease in:
Ulcerative colitis
Regional enteritis
� Nervous System
Acute exacerbations of multiple sclerosis
� Miscellaneous
Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy
Trichinosis with neurologic or myocardial involvement
PREDNISONE CONTRAINDICATIONS
WARNINGS
Usage in Pregnancy
While on corticosteroid therapy patients should not be vaccinated against smallpox. Other immunization procedures should not be undertaken in patients who are on corticosteroids, especially on high dose, because of possible hazards of neurological complications and a lack of antibody response.
PRECAUTIONS
GeneralDrug-induced secondary adrenocortical insufficiency may be minimized by gradula reduction of dosage. This type of relative isufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
DOSAGE AND ADMINISTRATION.)
PREDNISONE ADVERSE REACTIONS
Fluid and Electrolyte Disturbances:Sodium retention
Fluid retention
Congestive heart failure in susceptible patients
Potassium loss
Hypokalemic alkalosis
Hypertension
Musculoskeletal:
Muscle weakness
Steroid myopathy
Loss of muscle mass
Osteoporosis
Vertebral compression fractures
Aseptic necrosis of femoral and humeral heads
Pathologic fracture of long bones
Gastrointestinal:
Peptic ulcer with possible perforation and hemorrhage
Pancreatitis
Abdominal distention
Ulcerative esophagitis
Dermatologic:
Impaired wound healing
Thin fragile skin
Petechiae and ecchymoses
Facial erythema
Increased sweating
May suppress reactions to skin tests
Neurological:
Convulsions
Increased intracranial pressure with papilledema (pseudotumor cerebri) usually after treatment
Vertigo
Headache
Endocrine:
Menstrual irregularities
Development of Cushingoid state
Suppression of growth in children
Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery or illness
Decreased carbohydrate tolerance
Manifestations of latent diabetes mellitus
Increased requirements for insulin or oral hypoglycemic agents in diabetics
Ophthalmic:
Posterior subcapsular cataracts
Increased intraocular pressure
Glaucoma
Exophthalmos
Metabolic:
Negative nitrogen balance due to protein
Additional Reactions:
Urticaria and other allergic, anaphylactic or hypersensitivity reactions
www.fda.gov/medwatch.
DOSAGE & ADMINISTRATION
IT SHOULD BE EMPHASIZED THAT DOSAGE REQUIREMENTS ARE VARIABLE AND MUST BE INDIVIDUALIZED ON THE BASIS OF THE DISEASE UNDER TREATMENT AND THE RESPONSE OF THE PATIENT.
Multiple Sclerosis
ADT(Alternate Day Therapy)
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PREDNISONEPREDNISONE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!