Primidone
Primidone Tablet USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRIMIDONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PRIMIDONE INDICATIONS AND USAGE
- PRIMIDONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PRIMIDONE ADVERSE REACTIONS
- PRIMIDONE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Anticonvulsant
PRIMIDONE DESCRIPTION
Chemical name: 5-ethyldihydro- 5-phenyl-4,6 (1H, 5H) pyrimidinedione.
Structural formula:
C12H14N2O2
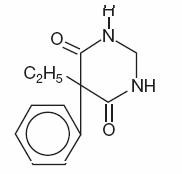 M.W. 218.25
M.W. 218.25
Primidone is a white crystalline powder, M.P. 279-284°C. It is very slightly soluble in methanol and methylene dichloride, slightly soluble in alcohol and insoluble in water. It possesses no acidic properties, in contrast to its barbiturate analog.
Primidone tablets 50 mg and 250 mg contain the following inactive ingredients: lactose monohydrate, magnesium stearate, methyl cellulose, purified water, sodium lauryl sulphate, sodium starch glycolate and talc.
Primidone tablets 250 mg also contain yellow iron oxide.
CLINICAL PHARMACOLOGY
Primidone raises electro- or chemoshock seizure thresholds or alters seizure patterns in experimental animals. The mechanism(s) of primidone’s antiepileptic action is not known.
Primidone per se has anticonvulsant activity as do its two metabolites, phenobarbital and phenylethylmalonamide (PEMA). In addition to its anticonvulsant activity, PEMA potentiates the anticonvulsant activity of phenobarbital in experimental animals.
PRIMIDONE INDICATIONS AND USAGE
Primidone tablet, used alone or concomitantly with other anticonvulsants, is indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy.
PRIMIDONE CONTRAINDICATIONS
Primidone tablet is contraindicated in:
1) patients with porphyria and
2) patients who are hypersensitive to phenobarbital (see CLINICAL PHARMACOLOGY ).
WARNINGS
The abrupt withdrawal of antiepileptic medication may precipitate status epilepticus. The therapeutic efficacy of a dosage regimen takes several weeks before it can be assessed.
Antiepileptic drugs (AEDs), including primidone, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1 Risk by indication for antiepileptic drugs in the pooled analysis
| Indication | Placebo Patientswith Events Per1000 Patients | Drug Patientswith Events Per1000 Patients | Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing primidone or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Usage in Pregnancy
To provide information regarding the effects of in utero exposure to primidone, physicians are advised to recommend that pregnant patients taking primidone enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
The effects of primidone in human pregnancy and nursing infants are unknown.
Recent reports suggest an association between the use of anticonvulsant drugs by women with epilepsy and an elevated incidence of birth defects in children born to these women. Data are more extensive with respect to diphenylhydantoin and phenobarbital, but these are also the most commonly prescribed anticonvulsants; less systematic or anecdotal reports suggest a possible similar association with the use of all known anticonvulsant drugs.
The reports suggesting an elevated incidence of birth defects in children of drugtreated epileptic women cannot be regarded as adequate to prove a definite cause and effect relationship.
There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; the possibility also exists that other factors leading to birth defects, e.g., genetic factors or the epileptic condition itself, may be more important than drug therapy. The great majority of mothers on anticonvulsant medication deliver normal infants. It is important to note that anticonvulsant drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorders are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus.
The prescribing physician will wish to weigh these considerations in treating or counseling epileptic women of childbearing potential. Neonatal hemorrhage, with a coagulation defect resembling vitamin K deficiency, has been described in newborns whose mothers were taking primidone and other anticonvulsants. Pregnant women under anticonvulsant therapy should receive prophylactic vitamin K1 therapy for one month prior to, and during, delivery.
PRECAUTIONS
The total daily dosage should not exceed 2 g. Since primidone therapy generally extends over prolonged periods, a complete blood count and a sequential multiple analysis-12 (SMA-12) test should be made every six months.
In Nursing Mothers
There is evidence that in mothers treated with primidone, the drug appears in the milk in substantial quantities. Since tests for the presence of primidone in biological fluids are too complex to be carried out in the average clinical laboratory, it is suggested that the presence of undue somnolence and drowsiness in nursing newborns of primidone-treated mothers be taken as an indication that nursing should be discontinued.
Suicidal Thinking and Behavior- Patients, their caregivers, and families should be counseled that AEDs, including primidone, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Patients should be encouraged to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 (see Usage in Pregnancy section ).
PRIMIDONE ADVERSE REACTIONS
The most frequently occurring early side effects are ataxia and vertigo. These tend to disappear with continued therapy, or with reduction of initial dosage. Occasionally, the following have been reported: nausea, anorexia, vomiting, fatigue, hyperirritability, emotional disturbances, sexual impotency, diplopia, nystagmus, drowsiness and morbilliform skin eruptions. Granulocytopenia, agranulocytosis, and redcell hypoplasia and aplasia, have been reported rarely. These and, occasionally, other persistent or severe side effects may necessitate withdrawal of the drug. Megaloblastic anemia may occur as a rare idiosyncrasy to primidone and to other anticonvulsants. The anemia responds to folic acid without necessity of discontinuing medication.
PRIMIDONE DOSAGE AND ADMINISTRATION
Adult Dosage
Patients 8 years of age and older who have received no previous treatment may be started on primidone tablets according to the following regimen using either 50 mg or scored 250 mg primidone tablets:
Days 1 to 3: 100 to 125 mg at bedtime
Days 4 to 6: 100 to 125 mg b.i.d.
Days 7 to 9: 100 to 125 mg t.i.d.
Day 10 to maintenance: 250 mg t.i.d.
For most adults and children 8 years of age and over, the usual maintenance dosage is three to four 250 mg primidone tablets daily in divided doses (250 mg t.i.d. or q.i.d.). If required, an increase to five or six 250 mg tablets daily may be made but daily doses should not exceed 500 mg q.i.d.
| INITIAL:ADULTS AND CHILDREN OVER 8 | ||||||
|---|---|---|---|---|---|---|
| KEY:• = 50 mg tablet; ● = 250 mg tablet | ||||||
| DAY | 1 | 2 | 3 | 4 | 5 | 6 |
| AM | •• | •• | •• | |||
| NOON | ||||||
| PM | •• | •• | •• | •• | •• | •• |
| DAY | 7 | 8 | 9 | 10 | 11 | 12 |
| AM | •• | •• | •• | ● | Adjust to Maintenance | |
| NOON | •• | •• | •• | ● | ||
| PM | •• | •• | •• | ● | ||
Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations of primidone tablets may be necessary for optimal dosage adjustment. The clinically effective serum level for primidone tablets is between 5 to 12 μg/mL.
In Patients Already Receiving Other Anticonvulsants
Primidone tablets should be started at 100 to 125 mg at bedtime and gradually increased to maintenance level as the other drug is gradually decreased. This regimen should be continued until satisfactory dosage level is achieved for the combination, or the other medication is completely withdrawn. When therapy with primidone tablets alone is the objective, the transition from concomitant therapy should not be completed in less than two weeks.
Pediatric Dosage
For children under 8 years of age, the following regimen may be used:
Days 1 to 3: 50 mg at bedtime
Days 4 to 6: 50 mg b.i.d.
Days 7 to 9: 100 mg b.i.d.
Day 10 to maintenance: 125 mg t.i.d. to 250 mg t.i.d.
For children under 8 years of age, the usual maintenance dosage is 125 to 250 mg three times daily or, 10 to 25 mg/kg/day in divided doses.
HOW SUPPLIED
Primidone tablets USP, 50 mg are white to off white, round, flat, beveled edged uncoated tablets debossed “RDY” and “477” on the periphery on one side and breakline on other side. They are supplied in bottles of 30’s, 60’s, 100’s, 500’s and unit dose package of 100 (10 x 10).
Bottles of 30 NDC 55111-477-30
Bottles of 60 NDC 55111-477-60
Bottles of 100 NDC 55111-477-01
Bottles of 500 NDC 55111-477-05
Unit Dosage Package of 100 (10x10) NDC 55111-477-78
Primidone tablets USP, 250 mg are cream to yellow, round, flat, beveled edged uncoated tablets debossed “RDY” and “476” on the periphery on one side and breakline on other side. They are supplied in bottles of 30’s, 60’s, 100’s, 500’s and unit dose package of 100 (10 x 10).
Bottles of 30 NDC 55111-476-30
Bottles of 60 NDC 55111-476-60
Bottles of 100 NDC 55111-476-01
Bottles of 500 NDC 55111-476-05
Unit Dosage Package of 100 (10x10) NDC 55111-476-78
Store at 20°–25° C (68°–77° F) [See USP Controlled Room Temperature].
Dispense in a well-closed container with a child-resistant closure.
Rx only
Manufactured by:
Dr. Reddy’s Laboratories Limited
Bachepalli – 502 325 INDIA
Issued: 0609
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
Primidone Tablets USP, 50 mg - 100's Container
Primidone Tablets USP, 50 mg - Carton
Primidone Tablets USP, 250 mg - 100's Container

Primidone Tablets USP, 250 mg - Carton
PrimidonePrimidone TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrimidonePrimidone TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||