PrismaSol
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PrismaSol solution safely and effectively. See full prescribing information for PrismaSol solution. PrismaSol solution, for intravenous administration. Initial U.S. Approval: 2006INDICATIONS AND USAGEPrismaSol solution is indicated : As a replacement solution in Continuous Renal Replacement Therapy (CRRT) In case of drug poisoning when CRRT is used DOSAGE AND ADMINISTRATIONThe mode of therapy, solute formulations, flow rates and length of therapy should be selected by the physician responsible for managing treatment depending on the clinical condition of the patient as well as the patient's fluid, electrolyte, acid base and glucose balance.DOSAGE FORMS AND STRENGTHS1000 mL of the reconstituted PrismaSol solution contains (in mEq/L except where noted): BGK0/2.5 BGK 4/2.5 BGK 4/3.5 BGK 2/3.5 Ca2+ 2.5 2.5 3.5 3.5 HCO3 - 32 32 32 32 K+ 0 4.0 4.0 2.0 Mg2+ 1.5 1.5 1.0 1.0 Na+ 140 140 140 140 Cl- 109.0 113.0 113.5 111.5 Lactate 3.0 3.0 3.0 3.0 Dextrose 100mg/dL 100mg/dL 100 mg/dL 100mg/dL BGK 2/0 B22GK 4/0 BK 0/0/1.2 BGK4/0/1.2 Ca2+ 0 0 0 0 HCO3 - 32 22 32 32 K+ 2.0 4.0 0 4.0 Mg2+ 1.0 1.5 1.2 1.2 Na+ 140 140 140 140 Cl- 108.0 120.5 106.2 110.2 Lactate 3.0 3.0 3.0 3.0 Dextrose 100mg/dL 100mg/dL 0 100mg/dL CONTRAINDICATIONSNoneWARNINGS AND PRECAUTIONS The solution in compartment A must be mixed with the solution of compartment B before use. Due to chemical reasons, after removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment. Do not administer the reconstituted solution unless it is clear and free of visible particulate matter. The patient's hemodynamic fluid, electrolyte and acid-base balance should be monitored throughout the procedure. Abnormalities in the plasma concentration of potassium, calcium, and glucose may be corrected by the use of appropriate formulations of PrismaSol solution. Use only with continuous extra-corporeal blood purification equipment in CRRT. The solution may be heated to no more than 40°C/104°F inside of the overwrap and this must be carefully controlled. Side EffectsImproper use can lead to fluid imbalance and disturbances in electrolyte, acid-base and glucose balance To report SUSPECTED ADVERSE REACTIONS, contact Gambro at 1800-651-2623 / www.gambro.com or FDA 1-800-FDA-1088 or www.fda.gov/medwatch
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 INDICATION AND USAGE
- 2 PRISMASOL DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 PRISMASOL CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 PRISMASOL ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATION
- 10 OVERDOSAGE
- 11 PRISMASOL DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 16 HOW SUPPLIED STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 0/2.5
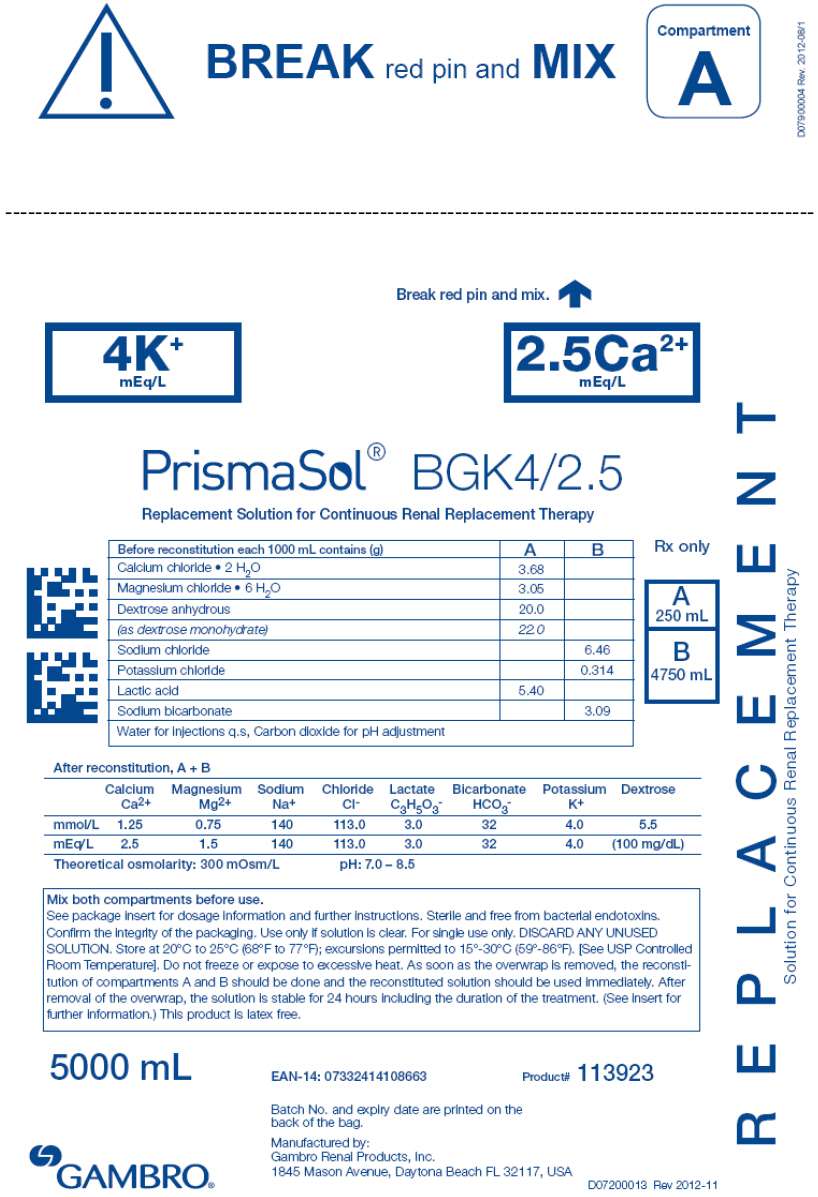
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/2.5
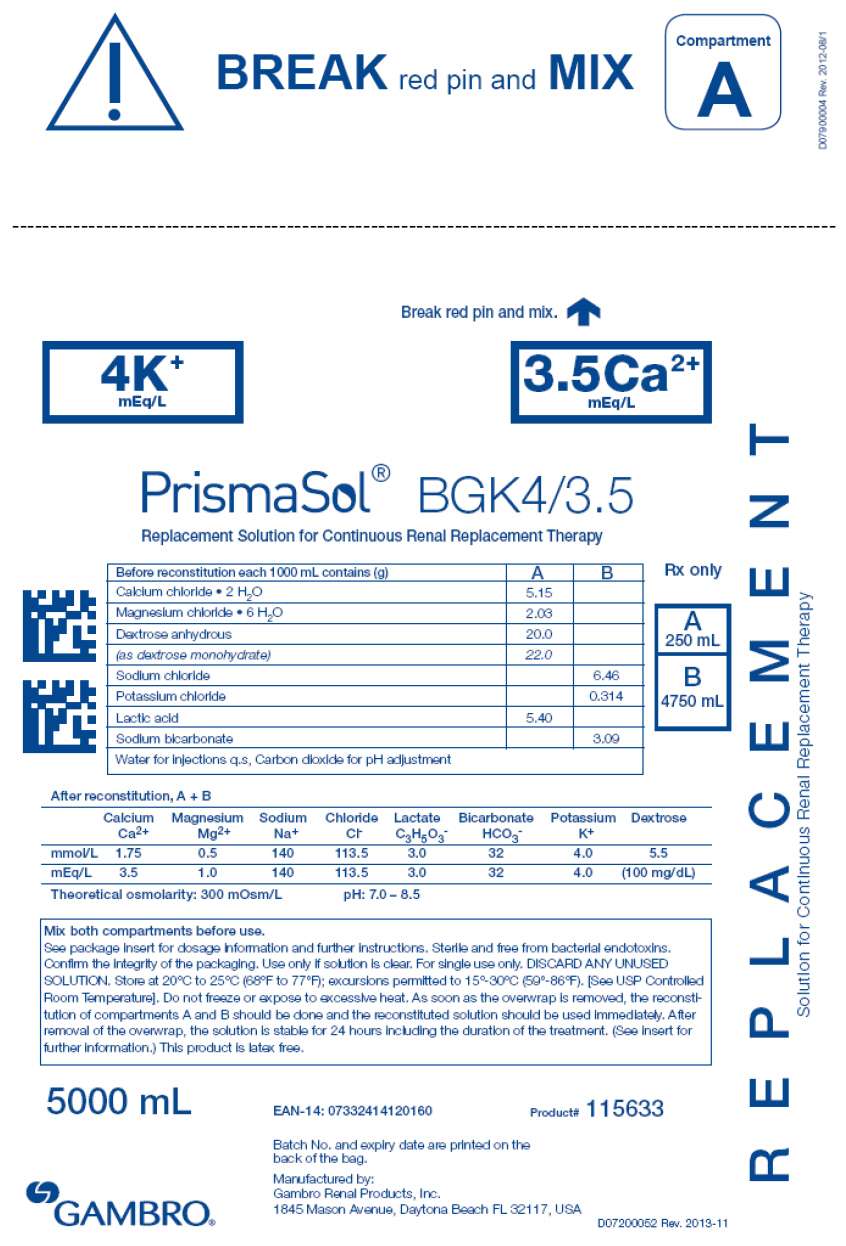
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/3.5
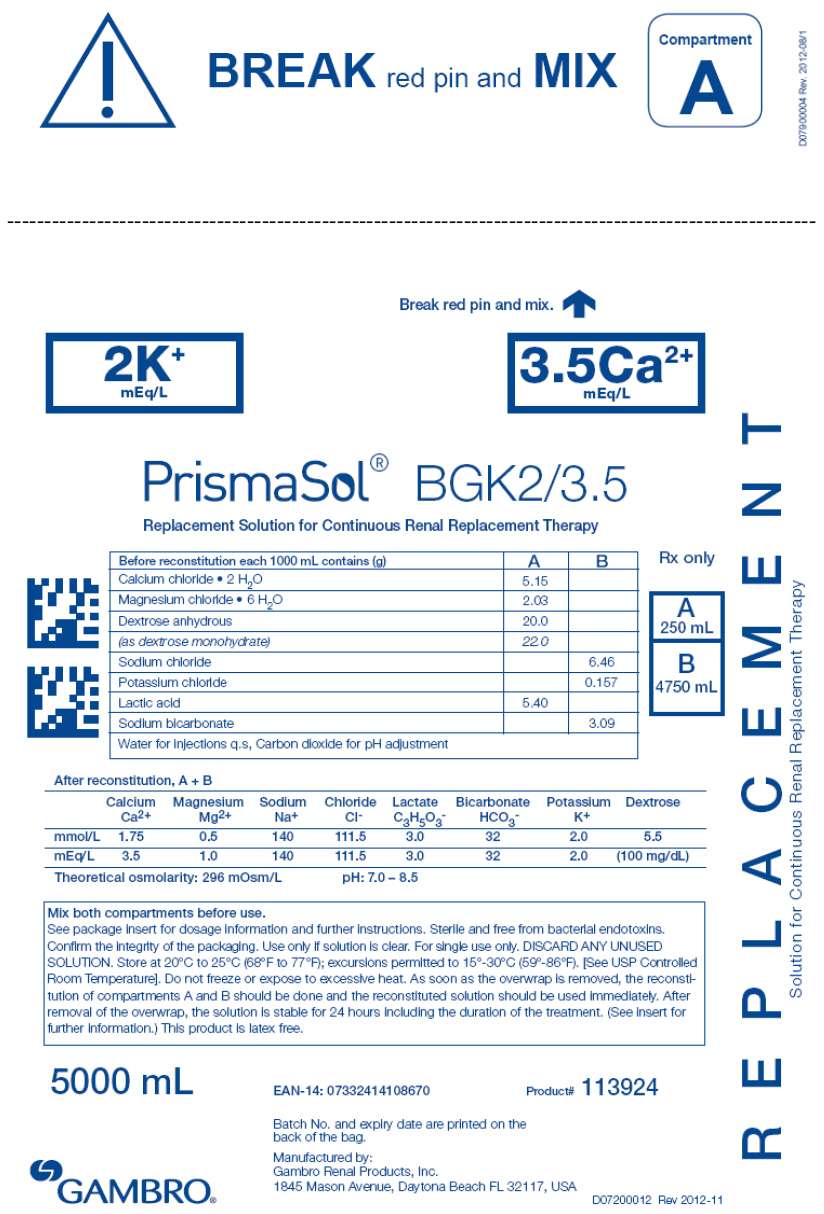
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 2/3.5
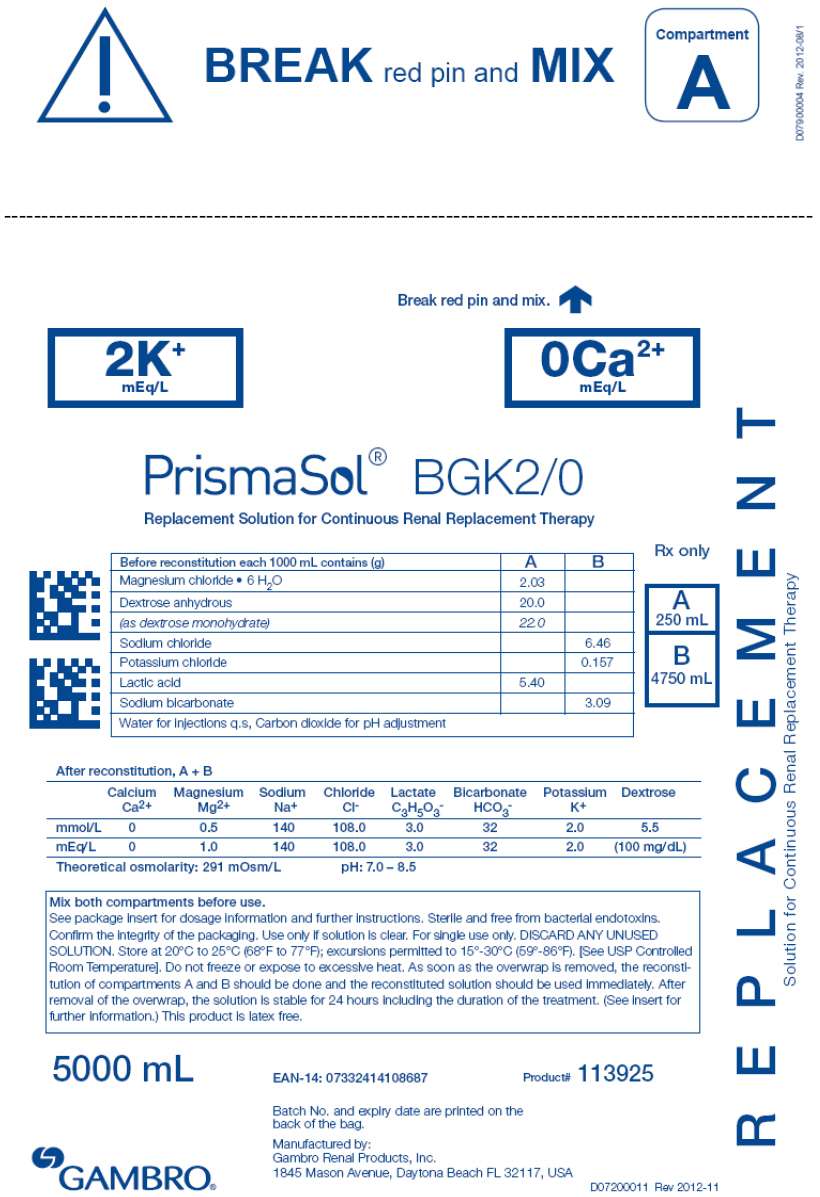
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 2/0
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/0
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 0/0/1.2
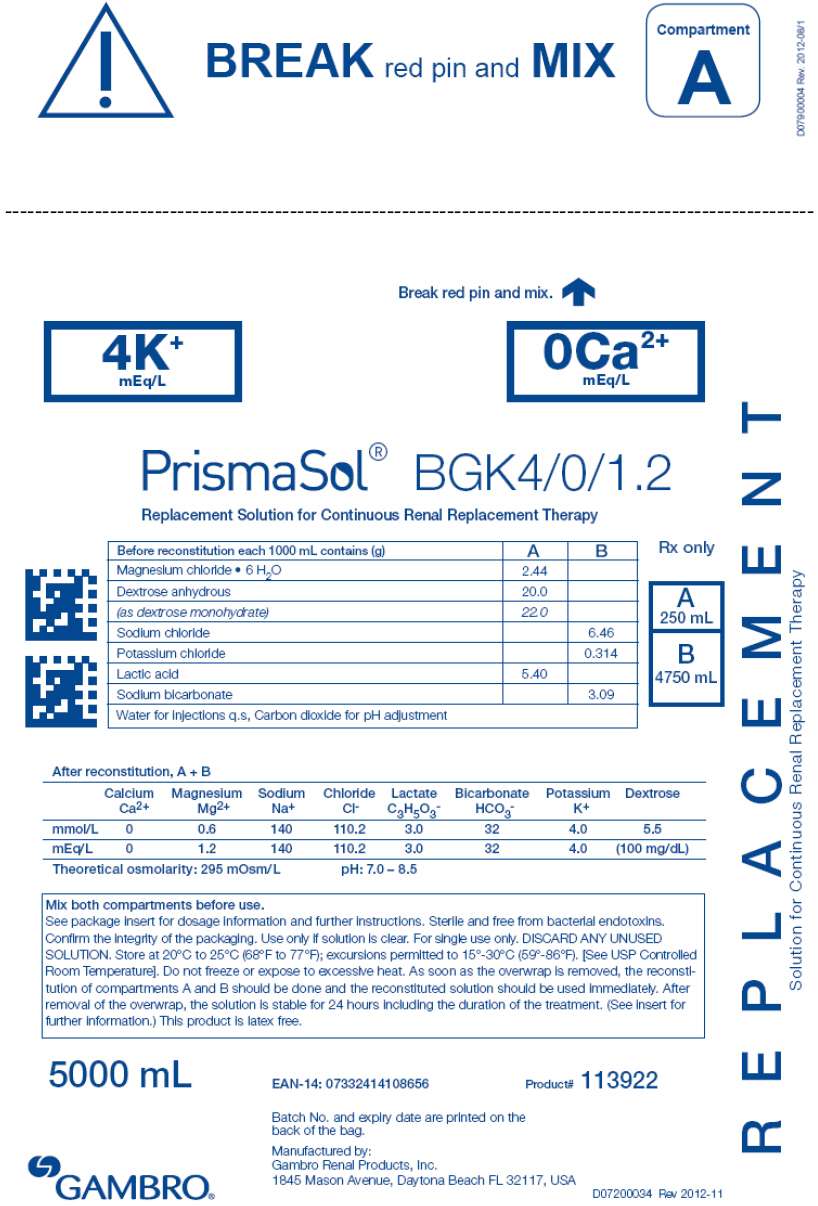
- PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/0/1.2
FULL PRESCRIBING INFORMATION
1 INDICATION AND USAGE
PrismaSol solution is indicated in adults and children for use as a replacement solution in Continuous Renal Replacement Therapy (CRRT) to replace plasma volume removed by ultrafiltration and to correct electrolytes and acid-base imbalances. PrismaSol solution may also be used in case of drug poisoning when CRRT is used to remove filterable substances.
2 DOSAGE AND ADMINISTRATION
2.1 Individualization of Treatments
The mode of therapy, solute formulation, flow rates and length of therapy should be selected by the physician responsible for managing treatment depending on the clinical condition of the patient as well as the patient's fluid, electrolyte, acid base and glucose balance. PrismaSol solution can be administered into the extra-corporeal circuit before (pre-dilution) and/or after the hemofilter or hemodiafilter (post-dilution).
In post-dilution hemofiltration, the replacement rate should not be greater than one third of the blood flow rate; e.g., for blood flow of 100 mL/min, equivalent to 6000 mL/hour, post-filter replacement rate should not exceed 2000 mL/hour.
2.2 Directions for use
PrismaSol solution should be inspected visually for particulate matter and discoloration prior to administration. Use only if the solution is clear and all seals are intact. Press bag firmly to test for any leakage. Do not use if container is damaged or leaking.
When connecting solution bags, follow the instructions in this leaflet for correct use of the access ports. Incorrect use of the access port or other restrictions to fluid flow might lead to incorrect patient weight loss and may result in machine alarms.
Continuing treatment without resolving the originating cause may result in patient injury or death.
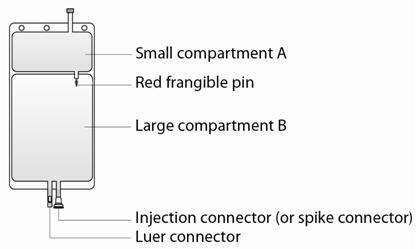
The electrolyte solution (small compartment A) is added to the buffer solution (large compartment B) by breaking the red frangible pin immediately before use and mixing the contents of compartments A and B.
- The reconstituted solution is for single patient use only
- Aseptic technique should be used throughout administration to the patient.
- Discard any unused solution.
As soon as the overwrap is removed, the reconstitution of compartments A and B should be done and the reconstituted solution should be used immediately.
Due to chemical reasons, after removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment.
|
|
Step 1 Immediately before use, remove the overwrap from the bag and mix the solutions in the two different compartments. Open the seal by breaking the red frangible pin between the two compartments of the bag. The frangible pin will remain in the bag. (See Figure 1 beside) |
|
|
Step 2 Make sure all the fluid from the small compartment A is transferred into the large compartment B. (See Figure 2 beside) Step 3 Rinse the small compartment A twice by pressing the mixed solution back into the small compartment A and then back into the large compartment B. (See Figure 3 beside) |
|
|
Step 4 When the small compartment A is empty: shake the large compartment B so that the contents mix completely. (See Figure 4 beside) The solution is now ready to use and the bag can be hung on the equipment. |
Step 5 The replacement line may be connected to the bag through either the luer connector or the injection connector (or spike connector).
|
|
Step 5a The luer connector is a needle-less and swabbable connector. Remove the cap with a twist and pull motion, and connect the male luer lock on the replacement line to the female luer receptor on the bag. (See Figure 5a beside) Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely during use. When the replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. |
|
|
Step 5b If the injection connector (or spike connector) is used, first remove the snap-off cap. Then introduce the replacement line spike through the rubber septum of the bag connector. (See Figure 5b beside) Ensure that the spike is fully inserted and verify that the fluid is flowing freely during use. |
Additions: The large compartment B is fitted with an injection connector (or spike connector) for the addition of drugs after reconstitution of the solution. When introducing additives, use aseptic techniques.
Phosphate: Phosphate up to 1.2 mmol/L may be added to the solution. If potassium phosphate is added, the total potassium concentration should not exceed 4 mEq/L.
Other drugs: Some drugs may be incompatible with PrismaSol solution. In general, other drugs should be administered through a different line.
3 DOSAGE FORMS AND STRENGTHS
| BGK0/2.5 | BGK4/2.5 | BGK4/3.5 | BGK2/3.5 | BGK2/0 | B22GK4/0 | BK0/0/1.2 | BGK4/0/1.2 |
Each PrismaSol solution represents of a unique combination of the active ingredients listed below.
BEFORE RECONSTITUTION
1000 mL of clear electrolyte solution (small compartment A) contains (g):
| Active ingredients | PrismaSol BGK0/2.5 |
PrismaSol BGK 4/2.5 |
PrismaSol BGK 4/3.5 |
PrismaSol BGK 2/3.5 |
PrismaSol BGK 2/0 |
PrismaSol B22GK4/0 |
PrismaSol BK 0/0/1.2 |
PrismaSol BGK4/0/1.2 |
|---|---|---|---|---|---|---|---|---|
| Calcium chloride • 2H2O | 3.68 | 3.68 | 5.15 | 5.15 | 0 | 0 | 0 | 0 |
| Magnesium chloride • 6H2O | 3.05 | 3.05 | 2.03 | 2.03 | 2.03 | 3.05 | 2.44 | 2.44 |
| Dextrose anhydrous | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 0 | 20.0 |
| (as dextrose monohydrate) | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 0 | 22.0 |
| Lactic acid | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 |
1000 mL of clear buffer solution (large compartment B) contains (g):
| Active ingredients | PrismaSol BGK0/2.5 |
PrismaSol BGK 4/2.5 |
PrismaSol BGK 4/3.5 |
PrismaSol BGK 2/3.5 |
PrismaSol BGK 2/0 |
PrismaSol B22GK4/0 |
PrismaSol BK 0/0/1.2 |
PrismaSol BGK4/0/1.2 |
|---|---|---|---|---|---|---|---|---|
| Sodium chloride | 6.46 | 6.46 | 6.46 | 6.46 | 6.46 | 7.07 | 6.46 | 6.46 |
| Sodium bicarbonate | 3.09 | 3.09 | 3.09 | 3.09 | 3.09 | 2.21 | 3.09 | 3.09 |
| Potassium chloride | 0 | 0.314 | 0.314 | 0.157 | 0.157 | 0.314 | 0 | 0.314 |
AFTER RECONSTITUTION of compartments A and B
1000 mL of the clear reconstituted solution contains:
| in mEq/L except where noted | PrismaSol BGK0/2.5 |
PrismaSol BGK 4/2.5 |
PrismaSol BGK4/3.5 |
PrismaSol BGK 2/3.5 |
PrismaSol BGK 2/0 |
PrismaSol B22GK4/0 |
PrismaSol BK 0/0/1.2 |
PrismaSol BGK4/0/1.2 |
|---|---|---|---|---|---|---|---|---|
|
Calcium Ca2+ |
2.5 | 2.5 | 3.5 | 3.5 | 0 | 0 | 0 | 0 |
|
Bicarbonate HCO3 - |
32 | 32 | 32 | 32 | 32 | 22 | 32 | 32 |
|
Potassium K+ |
0 | 4.0 | 4.0 | 2.0 | 2.0 | 4.0 | 0 | 4.0 |
|
Magnesium Mg2+ |
1.5 | 1.5 | 1.0 | 1.0 | 1.0 | 1.5 | 1.2 | 1.2 |
|
Sodium Na+ |
140 | 140 | 140 | 140 | 140 | 140 | 140 | 140 |
|
Chloride Cl- |
109.0 | 113.0 | 113.5 | 111.5 | 108.0 | 120.5 | 106.2 | 110.2 |
| Lactate | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Dextrose | 100mg/dL | 100mg/dL | 100 mg/dL | 100mg/dL | 100mg/dL | 100mg/dL | 0 | 100mg/dL |
| Theoretical Osmolarity | 292 mOsm/L |
300 mOsm/L |
300 mOsm/L |
296 mOsm/L |
291 mOsm/L |
296 mOsm/L |
282 mOsm/L |
295 mOsm/L |
4 CONTRAINDICATIONS
There is no known contraindication to the use of PrismaSol solution.
5 WARNINGS AND PRECAUTIONS
- The electrolyte solution contained in compartment A must be mixed with the buffer solution of compartment B before use in order to obtain the reconstituted solution suitable for hemofiltration /hemodiafiltration.
- Due to chemical reasons, after removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment.
- Do not administer the reconstituted solution unless it is clear and free of visible particulate matter.
- PrismaSol solution includes several formulations. Selection of a specific formulation depends on the patient's condition and treatment procedures.
- Administration of the solution should only be under the direction of a physician competent in intensive care treatment including CRRT.
- The patient's hemodynamic fluid, electrolyte and acid-base balance should be monitored throughout the procedure. Note that citrate, when used as an anticoagulant, contributes to the base load and can reduce plasma calcium levels.
- During hemofiltration or hemodiafiltration, abnormalities in the plasma concentration of potassium, calcium, and glucose may develop. These abnormalities may be corrected by the use of appropriate formulations of PrismaSol solution. Abnormalities in plasma phosphate concentration, especially hypophosphatemia, may also occur. Hypophosphatemia may require phosphate supplementation to maintain plasma concentrations in the physiologic range.
- Use only with continuous extra-corporeal blood purification equipment in CRRT.
- When connecting solution bags, follow the instructions in this leaflet for correct use of the access ports. Incorrect use of the access port or other restrictions to fluid flow might lead to incorrect patient weight loss and may result in machine alarms. Continuing treatment without resolving the originating cause may result in patient injury or death.
- The solution may be heated to no more than 40°C/104°F inside of the overwrap and this must be carefully controlled. After heating, verify that the solution remains clear and contains no particulate matter.
Diabetes Mellitus or Glucose Intolerance
Patients may require initiation of insulin therapy or modification of insulin dosage during treatment with PrismaSol solution. Appropriate monitoring of blood glucose should be performed and insulin dosage adjusted accordingly.
6 ADVERSE REACTIONS
Adverse reactions can result from the solution or the CRRT procedure.
Improper use can lead to fluid imbalance and disturbances in electrolyte, acid-base and glucose balance.
7 DRUG INTERACTIONS
As with the use of other replacement solutions, blood concentrations of filterable drugs may be influenced by CRRT.
The blood concentrations of certain drugs may need to be monitored and appropriate therapy implemented to correct for removal during treatment. In patients with cardiovascular disease, especially those using cardiac glycoside medications, plasma levels of calcium, potassium and magnesium must be carefully monitored.
8 USE IN SPECIFIC POPULATION
8.1 Pregnancy
Pregnancy Category C.
PrismaSol solution is a replacement solution of electrolytes, bicarbonate and dextrose and is pharmacologically inactive. Animal reproduction studies have not been conducted with PrismaSol solution. While there are no adequate and well controlled studies in pregnant women, appropriate administration of PrismaSol solution with monitoring of fluid, electrolyte, acid-base and glucose balance, is not expected to cause fetal harm, or affect reproductive capacity. Maintenance of normal acid-base balance is important for fetal well being.
8.3 Nursing Mothers
PrismaSol solution is a replacement solution of electrolytes, bicarbonate and dextrose and is pharmacologically inactive. The components of PrismaSol solution are excreted in human milk. Appropriate administration of PrismaSol solution with monitoring of fluid, electrolyte, acid-base and glucose balance, is not expected to harm a nursing infant.
8.4 Pediatric
Safety and effectiveness have been established based on published clinical data of CCRT replacement solutions used in adults and children. Two hemofiltration studies have shown that CRRT can be used to treat pediatric patients, including a study of newborns to 17 years olds. These pediatric studies utilized replacement solutions with a composition similar to PrismaSol solutions. No adequate and well-controlled studies have been conducted with PrismaSol solutions in pediatric patients.
8.5 Geriatric
Safety and effectiveness in geriatric patients have been reported in some studies on the general population. No formal specific study was carried out in the geriatric population.
Although clinical experience has not identified differences in responses between elderly and younger patients, greater sensitivity of some individuals cannot be ruled out.
10 OVERDOSAGE
Overdosage with PrismaSol solution should not occur if the procedure is carried out appropriately with careful monitoring of fluid, electrolyte, acid-base and glucose balance. However, overdosage can occur if fluid administration is in excess of the volume required to achieve the prescribed fluid balance or if delivery of a solute component in the solution exceeds a patient's needs. On the other hand, excessive fluid and solute removal may also occur in CRRT, potentially resulting in volume depletion and solute deficits.
The management of either an excess or deficiency state with respect to fluid, electrolyte and acid-base balance may involve modifications in the rate of administration and/or the composition of CRRT solutions. In conjunction with these changes, adjustments in the effluent (dialysate or ultrafiltrate) rate may need to occur.
11 DESCRIPTION
PrismaSol solution is a clear, sterile solution free of bacterial endotoxins. This solution is used in Continuous Renal Replacement Therapies (CRRT) as a replacement solution in hemofiltration and hemodiafiltration.
It contains no bacteriostatic or antimicrobial agents.
PrismaSol solution is packaged in a two-compartment bag. The small compartment A contains electrolytes and the large compartment B contains buffer. The final reconstituted solution (5000 mL) is obtained after breaking the red frangible pin between compartment A and B and mixing both solutions.
Calcium chloride, USP, is chemically designated calcium chloride dihydrate (CaCl2 • 2 H2O).
Magnesium chloride, USP, is chemically designated magnesium chloride hexahydrate (MgCl2 • 6H2O).
Dextrose, USP, is chemically designated D-Glucose anhydrous (C6H12O6) or D-Glucose monohydrate (C6H12O6 • H2O).
Lactic acid, USP, is chemically designated CH3CH(OH)COOH.
Sodium chloride, USP, is chemically designated NaCl.
Potassium chloride, USP, is chemically designated KCl.
Sodium bicarbonate, USP, is chemically designated NaHCO3.
The pH of the final solution is in the range of 7.0 to 8.5.
Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g. di 2-ethylhexyl phthalate (DEHP), up to 3 parts per million; however, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by in-vitro toxicity studies.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
PrismaSol solution is a pharmacologically inactive solution. The electrolyte concentrations in the PrismaSol solutions are chosen to restore plasma levels to clinically desired concentrations or maintain plasma levels at the desired concentrations.
PrismaSol solution is used as replacement solution to replace water and electrolytes removed during hemofiltration and hemodiafiltration. Bicarbonate in the solution is used as an alkalinizing buffer to normalize acid-base balance.
Lactate is used for the adjustment of the solution pH and is metabolized to bicarbonate.
When dextrose is present, it is intended to help normalize glucose balance.
12.2 Pharmacokinetics
The distribution of electrolytes, bicarbonate and dextrose is determined by the patient's clinical condition, metabolic status, and residual renal function.
The elimination and replacement of water, electrolytes and buffer depend on the patient's electrolyte and acid-base balance, metabolic status, residual renal function and ongoing physiologic losses through intestinal, respiratory and cutaneous routes.
16 HOW SUPPLIED STORAGE AND HANDLING
PrismaSol solution is supplied in a two compartment bag made of Polyvinyl chloride (PVC). The 5000 mL bag is composed of a small compartment (250 mL) and a large compartment (4750 mL). The two compartments are separated by a red frangible pin.
The bag is overwrapped with a transparent overwrap.
| Container | Fill Volume | NDC |
|---|---|---|
| PrismaSol BGK0/2.5 | 5000 mL | 24571-108-05 |
| PrismaSol BGK4/2.5 | 5000 mL | 24571-105-05 |
| PrismaSol BGK4/3.5 | 5000 mL | 24571-104-05 |
| PrismaSol BGK2/3.5 | 5000 mL | 24571-103-05 |
| PrismaSol BGK2/0 | 5000 mL | 24571-102-05 |
| PrismaSol B22GK4/0 | 5000 mL | 24571-111-05 |
| PrismaSol BK0/0/1.2 | 5000 mL | 24571-113-05 |
| PrismaSol BGK4/0/1.2 | 5000 mL | 24571-114-05 |
Not all formulations may be marketed.
Storage conditions
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature]
Do not freeze or expose to excessive heat. Do not use if precipitate has formed or if container seals have been damaged.
Manufactured by:
Gambro Renal Products, Inc
1845 Mason Avenue
Daytona Beach, FL 32117, USA
Gambro and PrimaSol are trademarks belonging to the Gambro Group.
PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 0/2.5
PrismaSol
® BGK0/2.5
Replacement Solution for Continuous Renal Replacement Therapy

PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/2.5
PrismaSol
® BGK4/2.5
Replacement Solution for Continuous Renal Replacement Therapy
PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/3.5
PrismaSol
® BGK4/3.5
Replacement Solution for Continuous Renal Replacement Therapy
PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 2/3.5
PrismaSol
® BGK2/3.5
Replacement Solution for Continuous Renal Replacement Therapy
PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 2/0
PrismaSol
® BGK2/0
Replacement Solution for Continuous Renal Replacement Therapy
PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/0
PrismaSol
® B22GK4/0
Replacement Solution for Continuous Renal Replacement Therapy

PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 0/0/1.2
PrismaSol
® BK0/0/1.2
Replacement Solution for Continuous Renal Replacement Therapy

PRINCIPAL DISPLAY PANEL - 5000 mL Bag Label - 4/0/1.2
PrismaSol
® BGK4/0/1.2
Replacement Solution for Continuous Renal Replacement Therapy
PrismaSolCalcium Chloride, Magnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, and Sodium Bicarbonate SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolCalcium Chloride, Magnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, and Potassium Chloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolCalcium Chloride, Magnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, and Potassium Chloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolMagnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, and Potassium Chloride SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolMagnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, and Potassium Chloride SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolMagnesium Chloride, Lactic Acid, Sodium Chloride, and Sodium Bicarbonate SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolMagnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, and Potassium Chloride SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PrismaSolCalcium Chloride, Magnesium Chloride, Dextrose monohydrate, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, and Potassium Chloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||