Proactiv Solution
Proactiv Solution Daily Protection Plus Sunscreen SPF 30
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive Ingredients
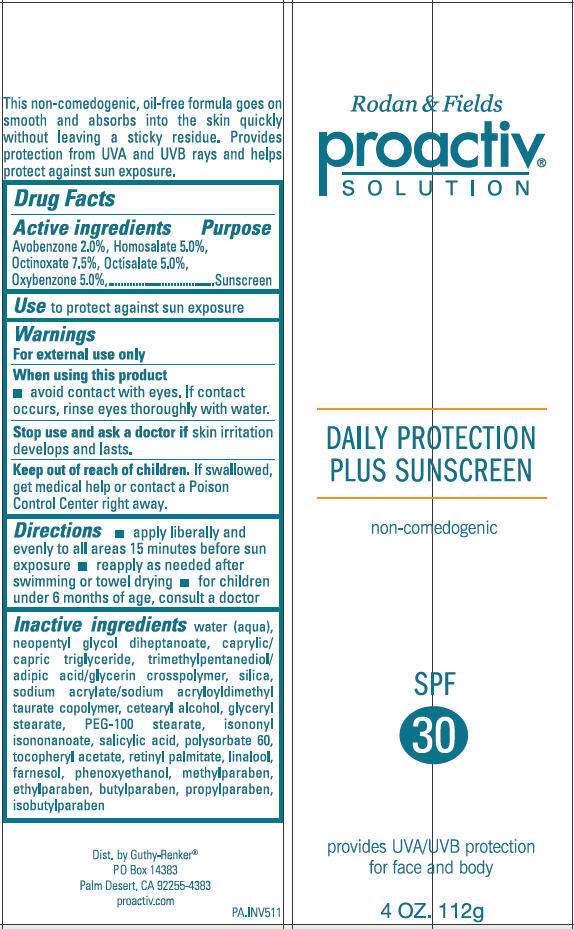
- PRINCIPAL DISPLAY PANEL - 112 g Tube Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Avobenzone 2.0%, Homosalate 5.0%, Octinoxate 7.5%, Octisalate 5.0%, Oxybenzone 5.0%
Purpose
Sunscreen
Use
To protect against sun exposure
Warnings
For external use only
When using this product
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if skin irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly to all areas 15 minutes before sun exposure
- Reapply as needed after swimming or towel drying.
- For children under 6 months of age, consult a doctor.
Inactive Ingredients
water, neopentyl glycol diheptanoate, caprylic/capric triglyceride, trimethylpentanediol/adipic acid/glycerin crosspolymer, silica, sodium acrylate/sodium acryloyldimethyl taurate copolymer, cetearyl alcohol, glyceryl stearate, PEG-100 stearate, isononyl isononanoate, salicylic acid, polysorbate 60, tocopheryl acetate, retinyl palmitate, linalool, farnesol, phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, isobutylparaben
Dist. By Guthy-Renker®
PO Box 14383
Palm Desert, CA 92255-4383
PRINCIPAL DISPLAY PANEL - 112 g Tube Label
Rodan & Fields
proactiv
®
SOLUTION
DAILY PROTECTION
PLUS SUNSCREEN
non-comedogenic
SPF
30
provides UVA/UVB protection
for face and body
4 OZ. 112g
Proactiv SolutionAVOBENZONE, HOMOSALATE, OCTINOXATE, OCTISALATE, and OXYBENZONE CREAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||