Prochlorperazine Maleate
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- PROCHLORPERAZINE MALEATE DESCRIPTION
- INDICATIONS & USAGE
- PROCHLORPERAZINE MALEATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PROCHLORPERAZINE MALEATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
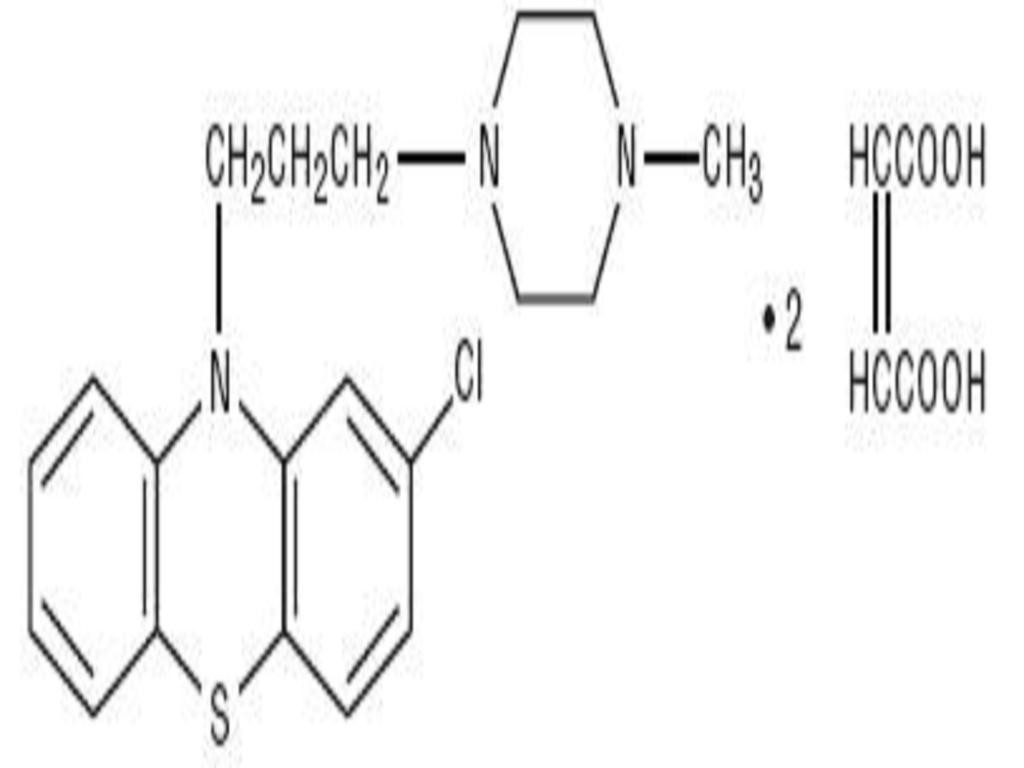
PROCHLORPERAZINE MALEATE DESCRIPTION
INDICATIONS & USAGE
PROCHLORPERAZINE MALEATE CONTRAINDICATIONS
WARNINGS
Increased Mortality in Elderly Patients With Dementia-Related PsychosisTardive Dyskinesia
Neuroleptic Malignant Syndrome (NMS)
Pregnancy
Non-teratogenic Effects
Nursing Mothers
PRECAUTIONS
Long-Term Therapy
Leukopenia, Neutropenia and Agranulocytosis
Geriatric Use
PROCHLORPERAZINE MALEATE ADVERSE REACTIONS
Extrapyramidal Reactions
Dystonia
Class effect
Motor Restlessness
Pseudo-parkinsonism
Tardive Dyskinesia
Adverse Reactions Reported With Prochlorperazine or Other Phenothiazine Derivatives
OVERDOSAGE
Symptoms
Treatment
DOSAGE & ADMINISTRATION
AdultsElderly Patients
1. To Control Severe Nausea and Vomiting
2. In Adult Psychiatric Disorders
Children
1. Severe Nausea and Vomiting in Children
2. In Children With Schizophrenia
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Prochlorperazine MaleateProchlorperazine Maleate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!