ProHance
Bracco Diagnostics Inc.
Bracco Diagnostics Inc.
ProHance® (Gadoteridol) Injection, 279.3 mg/mL
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
- PROHANCE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRIALS
- PROHANCE INDICATIONS AND USAGE
- PROHANCE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY
- PROHANCE ADVERSE REACTIONS
- OVERDOSAGE
- PROHANCE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
FULL PRESCRIBING INFORMATION
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs.
- The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended ProHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration (see WARNINGS).
PROHANCE DESCRIPTION
ProHance (Gadoteridol) Injection is a nonionic contrast medium for magnetic resonance imaging (MRI), available as a 0.5M sterile clear colorless to slightly yellow aqueous solution in vials and syringes for intravenous injection.
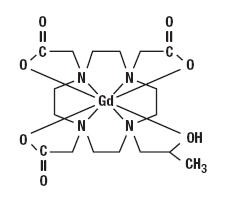
Gadoteridol is the gadolinium complex of 10-(2-hydroxy-propyl)-1,4,7,10- tetraazacyclododecane-1,4,7-triacetic acid with a molecular weight of 558.7, an empirical formula of C17H29N4O7Gd and has the following structural formula:
Each mL of ProHance contains 279.3 mg gadoteridol, 0.23 mg calteridol calcium, 1.21 mg tromethamine and water for injection. ProHance contains no antimicrobial preservative.
ProHance has a pH of 6.5 to 8.0. Pertinent physicochemical data are noted below:
| PARAMETER | ||
| Osmolality (mOsmol/kg water) | ||
| @ 37° C | 630 | |
| Viscosity | ||
| (cP) | @ 20° C | 2.0 |
| @ 37° C | 1.3 | |
| Specific Gravity | ||
| @ 25° C | 1.140 | |
| Density | ||
| (g/mL) | @ 25° C | 1.137 |
| Octanol: H2O coefficient | -3.68 ± 0.02 | |
ProHance has an osmolality 2.2 times that of plasma (285 mOsmol/kg water) and is hypertonic under conditions of use.
CLINICAL PHARMACOLOGY
Pharmacokinetics
The pharmacokinetics of intravenously administered gadoteridol in normal subjects conforms to a two-compartment open model with mean distribution and elimination half-lives (reported as mean ± SD) of about 0.20 ± 0.04 hours and 1.57 ± 0.08 hours, respectively.
Gadoteridol is eliminated in the urine with 94.4 ± 4.8% (mean ± SD) of the dose excreted within 24 hours post-injection. It is unknown if biotransformation or decomposition of gadoteridol occur in vivo.
The renal and plasma clearance rates (1.41 ± 0.33 mL/ min/kg and 1.50 ± 0.35 mL/ min/kg, respectively) of gadoteridol are essentially identical, indicating no alteration in elimination kinetics on passage through the kidneys and that the drug is essentially cleared through the kidney. The volume of distribution (204 ± 58 mL/kg) is equal to that of extracellular water, and clearance is similar to that of substances which are subject to glomerular filtration.
It is unknown if protein binding of ProHance occurs in vivo.
Pharmacodynamics
Gadoteridol is a paramagnetic agent and, as such, develops a magnetic moment when placed in a magnetic field. The relatively large magnetic moment produced by the paramagnetic agent results in a relatively large local magnetic field, which can enhance the relaxation rates of water protons in the vicinity of the paramagnetic agent.
In magnetic resonance imaging (MRI), visualization of normal and pathologic brain tissue depends in part on variations in the radiofrequency signal intensity that occur with 1) differences in proton density; 2) differences of the spin-lattice or longitudinal relaxation times (T1); and 3) differences in the spin-spin or transverse relaxation time (T2). When placed in a magnetic field, gadoteridol decreases T1 relaxation times in the target tissues. At recommended doses, the effect is observed with greatest sensitivity in the T1-weighted sequences.
Gadoteridol does not cross the intact blood-brain barrier and, therefore, does not accumulate in normal brain or in lesions that have a normal blood-brain barrier, e.g., cysts, mature post-operative scars, etc. However, disruption of the blood-brain barrier or abnormal vascularity allows accumulation of gadoteridol in lesions such as neoplasms, abscesses, and subacute infarcts. The pharmacokinetics of ProHance in various lesions is not known.
CLINICAL TRIALS
ProHance was evaluated in two blinded read trials in a total of 133 adults who had an indication for head and neck extracranial or extraspinal magnetic resonance imaging. These 133 adults (74 men, 59 women) had a mean age of 53 with a range of 19 to 76 years. Of these patients, 85% were Caucasian, 13% Black, 2% Asian, and < 1% other. The results of the non-contrast and gadoteridol MRI scans were compared. In this database, approximately 75-82% of the scans were enhanced. 45-48% of the scans provided additional diagnostic information, and 8-25% of the diagnoses were changed. The relevance of the findings to disease sensitivity and specificity has not been fully evaluated.
ProHance was evaluated in a multicenter clinical trial of 103 children who had an indication for a brain or spine MRI. These 103 children, (54 boys and 49 girls) had a mean age of 8.7 years with an age range of 2 to 20 years. Of these 103 children, 54 were between 2 and 12 years of age. Also, of these 103 children, 74% were Caucasian, 11% Black, 12% Hispanic, 2% Asian, and 2% other. The results of the non-contrast and gadoteridol MRI scans were compared. ProHance was given in one single 0.1 mmol/kg dose. Repeat dosing was not studied. In this database, MRI enhancement was noted in approximately 60% of the scans and additional diagnostic information in 30-95% of the scans.
PROHANCE INDICATIONS AND USAGE
Central Nervous System
ProHance (Gadoteridol) Injection is indicated for use in MRI in adults and children over 2 years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine and associated tissues.
Extracranial/Extraspinal Tissues
ProHance is indicated for use in MRI in adults to visualize lesions in the head and neck.
PROHANCE CONTRAINDICATIONS
None known.
WARNINGS
Nephrogenic Systemic Fibrosis (NSF)
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30-59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60-89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following ProHance administration to Bracco Diagnostics (1-800-257-8151) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch ).
Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended ProHance dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination. The usefulness of hemodialysis in the prevention of NSF is unknown (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION ).
Deoxygenated sickle erythrocytes have been shown in in vitro studies to align perpendicular to a magnetic field which may result in vaso-occlusive complications in vivo. The enhancement of magnetic moment by ProHance may possibly potentiate sickle erythrocyte alignment. ProHance in patients with sickle cell anemia and other hemoglobinopathies has not been studied.
Patients with other hemolytic anemias have not been adequately evaluated following administration of ProHance to exclude the possibility of increased hemolysis.
Patients with a history of allergy, drug reactions or other hypersensitivity-like disorders should be closely observed during the procedure and for several hours after drug administration. (See PRECAUTIONS-General ).
PRECAUTIONS
General
Gadoteridol is cleared from the body by glomerular filtration. The hepato-biliary enteric pathway of excretion has not been demonstrated with ProHance®. Dose adjustments in renal or hepatic impairment have not been studied. Therefore, caution should be exercised in patients with either renal or hepatic impairment.
In a patient with a history of grand mal seizure, the possibility to induce such a seizure by ProHance® is unknown.
The possibility of a reaction, including serious, life threatening, or fatal, anaphylactic or cardiovascular reactions, or other idiosyncratic reactions (see ADVERSE REACTIONS ), should always be considered, especially in those patients with a history of a known clinical hypersensitivity or a history of asthma or other allergic respiratory disorders.
Diagnostic procedures that involve the use of contrast agents should be carried out under direction of a physician with the prerequisite training and a thorough knowledge of the procedure to be performed.
When ProHance (Gadoteridol) Injection is to be injected using nondisposable equipment, scrupulous care should be taken to prevent residual contamination with traces of cleansing agents. After ProHance is drawn into a syringe, the solution should be used immediately.
Repeat Procedures: Repeated procedures have not been studied. Sequential use during the same diagnostic session has only been studied in central nervous system use. (See Pharmacokinetics under CLINICAL PHARMACOLOGY and Central Nervous System under DOSAGE AND ADMINISTRATION).
Information for patients:
Patients scheduled to receive ProHance should be instructed to inform their physician if the patient;
- is pregnant or breast feeding
- has anemia or diseases that affect the red blood cells
- has a history of renal or hepatic disease, seizure, hemoglobinopathies, asthma or allergic respiratory diseases.
- has recently received a GBCA.
GBCAs increase the risk for NSF among patients with impaired elimination of the drugs. To counsel patients at risk for NSF:
- Describe the clinical manifestations of NSF
- Describe procedures to screen for the detection of renal impairment
Instruct the patients to contact their physician if they develop signs or symptoms of NSF following ProHance administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness.
CARCINOGENESIS, MUTAGENESIS, AND IMPAIRMENT OF FERTILITY
No animal studies have been performed to evaluate the carcinogenic potential of gadoteridol or potential effects on fertility.
ProHance did not demonstrate genotoxic activity in bacterial reverse mutation assays using Salmonella typhimurium and Escherichia coli, in a mouse lymphoma forward mutation assay, in an in vitro cytogenetic assay measuring chromosomal aberration frequencies in Chinese hamster ovary cells, nor in an in vivo mouse micronucleus assay at intravenous doses up to 5.0 mmol/kg.
Pregnancy Category C
ProHance administered to rats at 10 mmol/kg/day (33 times the maximum recommended human dose of 0.3 mmol/kg or 6 times the human dose based on a mmol/m2 comparison) for 12 days during gestation doubled the incidence of postimplantation loss. When rats were administered 6.0 or 10.0 mmol/ kg/day for 12 days, an increase in spontaneous locomotor activity was observed in the offspring. ProHance increased the incidence of spontaneous abortion and early delivery in rabbits administered 6 mmol/kg/day (20 times the maximum recommended human dose or 7 times the human dose based on a mmol/m2 comparison) for 13 days during gestation.
There are no adequate and well-controlled studies in pregnant women. ProHance (Gadoteridol) Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ProHance is administered to a nursing woman.
Pediatric Use
Safety and efficacy in children under the age of 2 years have not been established. The safety and efficacy of doses > 0.1 mmol/kg; and sequential and/or repeat procedures has not been studied in children. (See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION sections)
PROHANCE ADVERSE REACTIONS
The adverse events described in this section were observed in clinical trials involving 1251 patients (670 males and 581 females). Adult patients ranged in age from 18-91 yrs. Pediatric patients ranged from 2-17 years. The racial breakdown was 83% Caucasian, 8% Black, 3% Hispanic, 2% Asian, and 1% other. In 2% of the patients, race was not reported.
The most commonly noted adverse experiences were nausea and taste perversion with an incidence of 1.4%. These events were mild to moderate in severity.
The following additional adverse events occurred in fewer than 1% of the patients:
| Body as a Whole: | Facial Edema; Neck Rigidity; Pain; Pain at Injection Site; Injection Site Reaction; Chest Pain; Headache; Fever; Itching; Watery Eyes; Abdominal Cramps; Tingling Sensation in Throat; Laryngismus; Flushed Feeling; Vasovagal Reaction; Anaphylactoid Reactions (characterized by cardiovascular, respiratory and cutaneous symptoms) |
| Cardiovascular: | Prolonged P-R Interval; Hypotension; Elevated Heart Rate; A-V Nodal Rhythm |
| Digestive: | Edematous and/or itching tongue; Gingivitis; Dry Mouth; Loose Bowel; Vomiting |
| Nervous System: | Anxiety; Dizziness; Paresthesia; Mental Status Decline; Loss of Coordination in Arm; Staring Episode; Seizure; Syncope |
| Respiratory System: | Dyspnea; Rhinitis; Cough |
| Skin and Appendages: | Pruritus; Rash; Rash Macular Papular; Urticaria; Hives; Tingling Sensation of Extremity and Digits |
| Special Senses: | Tinnitus |
The following adverse drug reactions have also been reported:
| Body as a Whole: | Generalized Edema; Laryngeal Edema; Malaise; Anaphylactoid Reactions (characterized by cardiovascular, respiratory and cutaneous symptoms, and rarely resulting in Death) |
| Cardiovascular: | Cardiac Arrest; Bradycardia; Hypertension; and Death in association with pre-existing cardiovascular disorders |
| Digestive: | Increased Salivation; Dysphagia |
| Nervous System: | Stupor; Tremor; Loss of Consciousness |
| Respiratory: | Apnea; Wheezing |
| Skin and Appendages: | Sweating; and Cyanosis |
| Special Senses: | Voice Alteration; Transitory Deafness |
| Urogenital: | Urinary Incontinence |
OVERDOSAGE
Clinical consequences of overdose with ProHance have not been reported.
PROHANCE DOSAGE AND ADMINISTRATION
Central Nervous System
ADULTS: The recommended dose of ProHance (Gadoteridol) Injection is 0.1 mmol/kg (0.2 mL/kg) administered as a rapid intravenous infusion (10 mL/min-60 mL/min) or bolus (> 60 mL/min). In patients with normal renal function suspected of having poorly enhancing lesions, in the presence of negative or equivocal scans, a supplementary dose of 0.2 mmol/kg (0.4 mL/kg) may be given up to 30 minutes after the first dose.
CHILDREN (2-18 years): The recommended dose of ProHance is 0.1 mmol/kg (0.2 mL/kg) administered as a rapid intravenous infusion (10 mL/min-60 mL/min) or bolus (> 60 mL/min). The safety and efficacy of doses > 0.1 mmol/kg, and sequential and/or repeat procedures has not been studied.
Extracranial/Extraspinal Tissues
ADULTS: The recommended dose of ProHance is 0.1 mmol/kg (0.2 mL/kg) administered as a rapid intravenous infusion (10 mL/min-60 mL/min) or bolus (> 60 mL/min).
CHILDREN: Safety and efficacy for extracranial/extra-spinal tissues has not been
established.
Dose adjustments in renal and liver impairment have not been studied.
To ensure complete injection of the contrast medium, the injection should be followed by a 5 mL normal saline flush. The imaging procedure should be completed within 1 hour of the first injection of ProHance (Gadoteridol) Injection.
Parenteral products should be inspected visually for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored or particulate matter is present. Any unused portion must be discarded in accordance with regulations dealing with the disposal of such materials.
HOW SUPPLIED
ProHance (Gadoteridol) Injection is a clear, colorless to slightly yellow solution containing 279.3 mg/mL of gadoteridol in rubber stoppered vials. ProHance is available in boxes of:
| Five 5 mL fills in single dose 15 mL vials | (NDC 0270-1111-04) |
| Five 10 mL fills in single dose 30 mL vials | (NDC 0270-1111-01) |
| Five 15 mL fills in single dose 30 mL vials | (NDC 0270-1111-02) |
| Five 20 mL fills in single dose 30 mL vials | (NDC 0270-1111-03) |
| Five 10 mL fills in single dose 20 mL prefilled syringes | (NDC 0270-1111-16) |
| Five 17 mL fills in single dose 20 mL prefilled syringes | (NDC 0270-1111-45) |
STORAGE
ProHance (Gadoteridol) Injection should be stored at 25°C (77°F) excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature]. Protect from light. DO NOT FREEZE. Should freezing occur in the vial, ProHance should be brought to room temperature before use. If allowed to stand at room temperature for a minimum of 60 minutes, ProHance (Gadoteridol) Injection should return to a clear, colorless to slightly yellow solution. Before use, examine the product to assure that all solids are redissolved and that the container and closure have not been damaged. Should solids persist, discard vial. Frozen syringes should be discarded.
Directions for Use of the ProHance® (Gadoteridol) Injection single dose syringe *
-
1) Screw the threaded tip of the plunger rod clockwise into the cartridge plunger and push forward a few millimeters to break any friction between the cartridge plunger and syringe barrel.
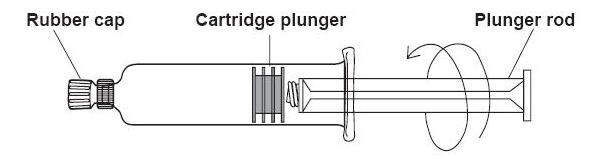
-
2) Holding syringe erect, aseptically remove the rubber cap from the tip of the syringe and attach either a sterile, disposable needle or tubing with a compatible luer lock using a push-twist action. -
3) Hold the syringe erect and push plunger forward until all of the air is evacuated and fluid either appears at the tip of the needle or the tubing is filled. Following the usual aspiration procedure, complete the injection. To ensure complete delivery of the contrast medium, the injection should be followed by a normal saline flush. -
4) Properly dispose of the syringe and any other materials used.
*The syringe assembly is a HYPAK SCF® single dose syringe supplied by Becton Dickinson.
This product is covered by one or more of:
U.S. Patent No. 4,885,363; U.S. Patent No. 4,963,344; U.S. Patent No. 5,474,756; U.S. Patent No. 5,846,519; and U.S. Patent No. 6,143,274.
Manufactured for
Bracco Diagnostics Inc.
Princeton, NJ 08543
by BIPSO GmbH
78224 Singen (Germany)
F.1/6058056
Revised July 2011
Prohance 5mL Vial label
NDC 0270-1111-04

Prohance 5mL Vial Box

Prohance 5x 10mL Syringe label
NDC 0270-1111-16
Prohance 10mL Syringe label

ProHancegadoteridol INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||