Propafenone HCl
McKesson Packaging Services a business unit of McKesson Corporation
Propafenone HydrochlorideTabletsRx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- PROPAFENONE HCL DESCRIPTION
- CLINICAL PHARMACOLOGY
- PROPAFENONE HCL INDICATIONS AND USAGE
- PROPAFENONE HCL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PROPAFENONE HCL ADVERSE REACTIONS
- OVERDOSAGE
- PROPAFENONE HCL DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
PROPAFENONE HCL DESCRIPTION
Propafenone hydrochloride is an antiarrhythmic drug. Propafenone has some structural similarities to beta-blocking agents. The structural formula of propafenone hydrochloride is given below:
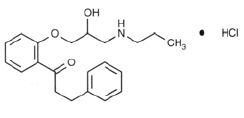
C21H27NO3·HCl M.W.=377.90
2’-[2-Hydroxy-3-(propylamino)propoxy]-3-phenylpropiophenone hydrochloride
Propafenone hydrochloride occurs as colorless crystals or white crystalline powder with a very bitter taste. It is slightly soluble in water (20°C), chloroform and ethanol. Each tablet, for oral administration, contains 150 mg or 225 mg of propafenone hydrochloride. The following inactive ingredients are contained in the tablet: colloidal silicon dioxide, croscarmellose sodium, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, pregelatinized starch, sodium lauryl sulfate, titanium dioxide and triacetin.
CLINICAL PHARMACOLOGY
Mechanism of Action
Propafenone hydrochloride is a Class 1C antiarrhythmic drug with local anesthetic effects, and a direct stabilizing action on myocardial membranes. The electrophysiological effect of propafenone hydrochloride manifests itself in a reduction of upstroke velocity (Phase 0) of the monophasic action potential. In Purkinje fibers, and to a lesser extent myocardial fibers, propafenone hydrochloride reduces the fast inward current carried by sodium ions. Diastolic excitability threshold is increased and effective refractory period prolonged. Propafenone reduces spontaneous automaticity and depresses triggered activity.
Studies in anesthetized dogs and isolated organ preparations show that propafenone has beta-sympatholytic activity at about 1/50 the potency of propranolol. Clinical studies employing isoproterenol challenge and exercise testing after single doses of propafenone indicate a beta-adrenergic blocking potency (per mg) about 1/40 that of propranolol in man. In clinical trials, resting heart rate decreases of about 8% were noted at the higher end of the therapeutic plasma concentration range. At very high concentrations in vitro, propafenone can inhibit the slow inward current carried by calcium but this calcium antagonist effect probably does not contribute to antiarrhythmic efficacy. Propafenone has local anesthetic activity approximately equal to procaine.
Electrophysiology
Electrophysiology studies in patients with ventricular tachycardia have shown that propafenone prolongs atrioventricular (AV) conduction while having little or no effect on sinus node function. Both AV nodal conduction time (AH interval) and His-Purkinje conduction time (HV interval) are prolonged. Propafenone has little or no effect on the atrial functional refractory period, but AV nodal functional and effective refractory periods are prolonged. In patients with Wolff Parkinson-White (WPW), propafenone reduces conduction and increases the effective refractory period of the accessory pathway in both directions. Propafenone slows conduction and consequently produces dose-related changes in the PR interval and QRS duration. QTc interval does not change.
| 337.5 mg | 450 mg | 675 mg | 900 mg | |||||
| Interval | msec | % | msec | % | msec | % | msec | % |
| *Change and percent change based on mean baseline values for each treatment group. | ||||||||
| RR | -14.5 | -1.8 | 30.6 | 3.8 | 31.5 | 3.9 | 41.7 | 5.1 |
| PR | 3.6 | 2.1 | 19.1 | 11.6 | 28.9 | 17.8 | 35.6 | 21.9 |
| QRS | 5.6 | 6.4 | 5.5 | 6.1 | 7.7 | 8.4 | 15.6 | 17.3 |
| QTC | 2.7 | 0.7 | -7.5 | -1.8 | 5.0 | 1.2 | 14.7 | 3.7 |
In any individual patient, the above ECG changes cannot be readily used to predict either efficacy or plasma concentration.
Propafenone hydrochloride causes a dose-related and concentration-related decrease in the rate of single and multiple PVCs and can suppress recurrence of ventricular tachycardia. Based on the percent of patients attaining substantial (80 to 90%) suppression of ventricular ectopic activity, it appears that trough plasma levels of 0.2 to 1.5 mcg/mL can provide good suppression, with higher concentrations giving a greater rate of good response.
When 600 mg/day propafenone was administered to patients with paroxysmal atrial tachyarrhythmias, mean heart rate during arrhythmia decreased 14 beats/min and 37 beats/min for paroxysmal atrial fibrillation/flutter (PAF) patients and paroxysmal supraventricular tachycardia (PSVT) patients, respectively.
Hemodynamics
Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by the beta blockade produced by propafenone may in itself aggravate congestive heart failure.
Additionally, like other Class 1C antiarrhythmic drugs, studies in humans have shown that propafenone exerts a negative inotropic effect on the myocardium. Cardiac catheterization studies in patients with moderately impaired ventricular function (mean C.I.=2.61 L/min/m2) utilizing intravenous propafenone infusions (2 mg/kg over 10 min + 2 mg/min for 30 min) that gave mean plasma concentrations of 3 mcg/mL (well above the therapeutic range of 0.2 to 1.5 mcg/mL) showed significant increases in pulmonary capillary wedge pressure, systemic and pulmonary vascular resistances and depression of cardiac output and cardiac index.
Pharmacokinetics and Metabolism
Propafenone hydrochloride is nearly completely absorbed after oral administration with peak plasma levels occurring approximately 3.5 hours after administration in most individuals. Propafenone exhibits extensive saturable presystemic biotransformation (first pass effect) resulting in a dose dependent and dosage form dependent absolute bioavailability; e.g., a 150 mg tablet had absolute bioavailability of 3.4%, while a 300 mg tablet had absolute bioavailability of 10.6%. A 300 mg solution which was rapidly absorbed, had absolute bioavailability of 21.4%. At still larger doses, above those recommended, bioavailability increases still further. Decreased liver function also increases bioavailability; bioavailability is inversely related to indocyanine green clearance reaching 60 to 70% at clearances of 7 mL/min and below. The clearance of propafenone is reduced and the elimination half-life increased in patients with significant hepatic dysfunction (see PRECAUTIONS).
Propafenone follows a nonlinear pharmacokinetic disposition presumably due to saturation of first pass hepatic metabolism as the liver is exposed to higher concentrations of propafenone and shows a very high degree of interindividual variability. For example, for a three-fold increase in daily dose from 300 to 900 mg/day there is a ten-fold increase in steady-state plasma concentration. The top 25% of patients given 375 mg/day, however, had a mean concentration of propafenone larger than the bottom 25%, and about equal to the second 25% of patients given a dose of 900 mg. Although food increased peak blood level and bioavailability in a single dose study, during multiple dose administration of propafenone to healthy volunteers food did not change bioavailability significantly.
There are two genetically determined patterns of propafenone metabolism. In over 90% of patients, the drug is rapidly and extensively metabolized with an elimination half-life from 2 to 10 hours. These patients metabolize propafenone into two active metabolites: 5-hydroxypropafenone which is formed by CYP2D6 and N-depropylpropafenone which is formed by both CYP3A4 and CYP1A2.
In vitro preparations have shown these two metabolites to have antiarrhythmic activity comparable to propafenone but in man they both are usually present in concentrations less than 20% of propafenone. Nine additional metabolites have been identified, most only in trace amounts. It is the saturable hydroxylation pathway that is responsible for nonlinear pharmacokinetic disposition.
In less than 10% of patients (and in any patient also receiving quinidine, see PRECAUTIONS), metabolism of propafenone is slower because the 5-hydroxy metabolite is not formed or is minimally formed. The estimated propafenone elimination half-life ranges from 10 to 32 hours. Decreased ability to form the 5-hydroxy metabolite of propafenone is associated with a diminished ability to metabolize debrisoquine and a variety of other drugs (encainide, metoprolol, dextromethorphan). In these patients, the N-depropylpropafenone occurs in quantities comparable to the levels occurring in extensive metabolizers. In slow metabolizers propafenone pharmacokinetics are linear.
There are significant differences in plasma concentrations of propafenone in slow and extensive metabolizers, the former achieving concentrations 1.5 to 2 times those of the extensive metabolizers at daily doses of 675 to 900 mg/day. At low doses the differences are greater, with slow metabolizers attaining concentrations more than five times that of extensive metabolizers. Because the difference decreases at high doses and is mitigated by the lack of the active 5-hydroxy metabolite in the slow metabolizers, and because steady-state conditions are achieved after 4 to 5 days of dosing in all patients, the recommended dosing regimen is the same for all patients. The greater variability in blood levels require that the drug be titrated carefully in patients with close attention paid to clinical and ECG evidence of toxicity (see DOSAGE AND ADMINISTRATION).
In vitro and in vivo studies have shown that the R-isomer of propafenone is cleared faster than the S-isomer via the 5-hydroxylation pathway (CYP2D6). This results in a higher ratio of S-propafenone during steady state.
Clinical Trials
In two randomized, crossover, placebo-controlled, double-blind trials of 60-90 days duration in patients with paroxysmal supraventricular arrhythmias PAF, or PSVT, propafenone reduced the rate of both arrhythmias, as shown in the following table:
| Study 1 | Study 2 | |||
| Propafenne | Placebo | Propafenone | Placebo | |
| PAF | n=30 | n=30 | n=9 | n=9 |
| Percent attack free | 53% | 13% | 67% | 22% |
| Median time to first recurrence | >98 days | 8 days | 62 days | 5 days |
| PSVT | n=45 | n=45 | n=15 | n=15 |
| Percent attack free | 47% | 16% | 38% | 7% |
| Median time to first recurrence | >98 days | 12 days | 31 days | 8 days |
The patient population in the above trials was 50% male with a mean age of 57.3 years. Fifty percent of the patients had a diagnosis of PAF and 50% had PSVT. Eighty percent of the patients received 600 mg/day propafenone. No patient died in the above 2 studies.
In the U.S. long-term safety trials, 474 patients (mean age: 57.4 ± 14.5 years) with supraventricular arrhythmias [195 with PAF, 274 with PSVT and 5 with both PAF and PSVT] were treated up to 5 years (mean: 14.4 months) with propafenone. Fourteen of the patients died. When this mortality rate was compared to the rate in a similar patient population (n=194 patients; mean age: 43 ± 16.8 years) studied in an arrhythmia clinic, there was no age-adjusted difference in mortality. This comparison was not, however, a randomized trial and the 95% confidence interval around the comparison was large, such that neither a significant adverse or favorable effect could be ruled out.
PROPAFENONE HCL INDICATIONS AND USAGE
In patients without structural heart disease, propafenone is indicated to prolong the time to recurrence of
-
-
As with other agents, some patients with atrial flutter treated with propafenone have developed 1:1 conduction, producing an increase in ventricular rate. Concomitant treatment with drugs that increase the functional AV refractory period is recommended.
The use of propafenone in patients with chronic atrial fibrillation has not been evaluated. Propafenone should not be used to control ventricular rate during atrial fibrillation.
Propafenone hydrochloride tablets are also indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgement of the physician, are life-threatening. Because of the proarrhythmic effects of propafenone, its use with lesser ventricular arrhythmias is not recommended, even if patients are symptomatic, and any use of the drug should be reserved for patients in whom, in the opinion of the physician, the potential benefits outweigh the risks.
Initiation of propafenone treatment, as with other antiarrhythmics used to treat life-threatening ventricular arrhythmias, should be carried out in the hospital.
Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular or atrial arrhythmias.
PROPAFENONE HCL CONTRAINDICATIONS
Propafenone hydrochloride tablets are contraindicated in the presence of uncontrolled congestive heart failure, cardiogenic shock, sinoatrial, atrioventricular and intraventricular disorders of impulse generation and/or conduction (e.g., sick sinus node syndrome, atrioventricular block) in the absence of an artificial pacemaker, bradycardia, marked hypotension, bronchospastic disorders, manifest electrolyte imbalance, and known hypersensitivity to the drug.
WARNINGS
Mortality
In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than six days but less than two years previously, an increased rate of death or reversed cardiac arrest rate (7.7%; 56/730) was seen in patients treated with encainide or flecainide (Class 1C antiarrhythmics) compared with that seen in patients assigned to a placebo (3%; 22/725). The average duration of treatment with encainide or flecainide in this study was ten months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) or other antiarrhythmic drugs is uncertain, but at present it is prudent to consider any 1C antiarrhythmic to have a significant risk in patients with structural heart disease. Given the lack of any evidence that these drugs improve survival, antiarrhythmic agents should generally be avoided in patients with non-life-threatening ventricular arrhythmias, even if the patients are experiencing unpleasant, but not life-threatening, symptoms or signs.
Proarrhythmic Effects
Propafenone hydrochloride, like other antiarrhythmic agents, may cause new or worsened arrhythmias. Such proarrhythmic effects range from an increase in frequency of PVCs to the development of more severe ventricular tachycardia, ventricular fibrillation or torsade de pointes; i.e., tachycardia that is more sustained or more rapid which may lead to fatal consequences. It is therefore essential that each patient given propafenone be evaluated electrocardiographically and clinically, prior to and during therapy, to determine whether the response to propafenone supports continued treatment.
Overall in clinical trials with propafenone, 4.7% of all patients had new or worsened ventricular arrhythmia possibly representing a proarrhythmic event (0.7% was an increase in PVCs; 4% a worsening, or new appearance, of VT or VF). Of the patients who had worsening of VT (4%), 92% had a history of VT and/or VT/VF, 71% had coronary artery disease, and 68% had a prior myocardial infarction. The incidence of proarrhythmia in patients with less serious or benign arrhythmias, which include patients with an increase in frequency of PVCs, was 1.6%. Although most proarrhythmic events occurred during the first week of therapy, late events also were seen and the CAST study (see above) suggests that an increased risk is present throughout treatment.
In the 474 patient U.S. multicenter trial in patients with symptomatic supraventricular tachycardia (SVT), 1.9% (9/474) of these patients experienced ventricular tachycardia (VT) or ventricular fibrillation (VF) during the study. However, in 4 of the 9 patients, the ventricular tachycardia was of atrial origin. Six of the nine patients that developed ventricular arrhythmias did so within 14 days of onset of therapy. About 2.3% (11/474) of all patients had a recurrence of SVT during the study which could have been a change in the patients’ arrhythmia behavior or could represent a proarrhythmic event. Case reports in patients treated with propafenone hydrochloride for atrial fibrillation/ flutter have included increased PVCs, VT, VF, and death.
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema)
PATIENTS WITH BRONCHOSPASTIC DISEASE SHOULD, IN GENERAL, NOT RECEIVE PROPAFENONE or other agents with beta-adrenergic-blocking activity.
Congestive Heart Failure
During treatment with oral propafenone in patients with depressed baseline function (mean EF=33.5%), no significant decreases in ejection fraction were seen. In clinical trial experience, new or worsened CHF has been reported in 3.7% of patients with ventricular arrhythmia; of those, 0.9% were considered probably or definitely related to propafenone. Of the patients with congestive heart failure probably related to propafenone, 80% had pre-existing heart failure and 85% had coronary artery disease. CHF attributable to propafenone developed rarely (<0.2%) in ventricular arrhythmia patients who had no previous history of CHF. CHF occurred in 1.9% of patients studied with PAF or PSVT.
As propafenone exerts both beta blockade and a (dose-related) negative inotropic effect on cardiac muscle, patients with congestive heart failure should be fully compensated before receiving propafenone. If congestive heart failure worsens, propafenone should be discontinued (unless congestive heart failure is due to the cardiac arrhythmia) and, if indicated, restarted at a lower dosage only after adequate cardiac compensation has been established.
Conduction Disturbances
Propafenone slows atrioventricular conduction and also causes first degree AV block. Average PR interval prolongation and increases in QRS duration are closely correlated with dosage increases and concomitant increases in propafenone plasma concentrations. The incidence of first degree, second degree, and third degree AV block observed in 2,127 ventricular arrhythmia patients was 2.5%, 0.6%, and 0.2%, respectively. Development of second or third degree AV block requires a reduction in dosage or discontinuation of propafenone. Bundle branch block (1.2%) and intraventricular conduction delay (1.1%) have been reported in patients receiving propafenone. Bradycardia has also been reported (1.5%). Experience in patients with sick sinus node syndrome is limited and these patients should not be treated with propafenone.
Effects on Pacemaker Threshold
Propafenone hydrochloride may alter both pacing and sensing thresholds of artificial pacemakers. Pacemakers should be monitored and programmed accordingly during therapy.
Hematologic Disturbances
Agranulocytosis (fever, chills, weakness, and neutropenia) has been reported in patients receiving propafenone. Generally, the agranulocytosis occurred within the first two months of propafenone therapy and upon discontinuation of therapy, the white count usually normalized by 14 days. Unexplained fever and/or decrease in white cell count, particularly during the initial three months of therapy, warrant consideration of possible agranulocytosis/granulocytopenia. Patients should be instructed to promptly report the development of any signs of infection such as fever, sore throat, or chills.
PRECAUTIONS
Hepatic Dysfunction
Propafenone is highly metabolized by the liver and should, therefore, be administered cautiously to patients with impaired hepatic function. Severe liver dysfunction increases the bioavailability of propafenone to approximately 70% compared to 3 to 40% for patients with normal liver function. In eight patients with moderate to severe liver disease, the mean half-life was approximately 9 hours. As a result, the dose of propafenone given to patients with impaired hepatic function should be approximately 20-30% of the dose given to patients with normal hepatic function (see DOSAGE AND ADMINISTRATION). Careful monitoring for excessive pharmacological effects (see OVERDOSAGE) should be carried out.
Renal Dysfunction
A considerable percentage of propafenone metabolites (18.5% to 38% of the dose/48 hours) are excreted in the urine.
Until further data are available, propafenone hydrochloride should be administered cautiously to patients with impaired renal function. These patients should be carefully monitored for signs of overdosage (see OVERDOSAGE).
Elevated ANA Titers
Positive ANA titers have been reported in patients receiving propafenone. They have been reversible upon cessation of treatment and may disappear even in the face of continued propafenone therapy. These laboratory findings were usually not associated with clinical symptoms, but there is one published case of drug-induced lupus erythematosis (positive rechallenge); it resolved completely upon discontinuation of therapy. Patients who develop an abnormal ANA test should be carefully evaluated and, if persistent or worsening elevation of ANA titers is detected, consideration should be given to discontinuing therapy.
Impaired Spermatogenesis
Reversible disorders of spermatogenesis have been demonstrated in monkeys, dogs and rabbits after high dose intravenous administration. Evaluation of the effects of short-term propafenone administration on spermatogenesis in 11 normal subjects suggests that propafenone produced a reversible, short-term drop (within normal range) in sperm count. Subsequent evaluations in 11 patients receiving propafenone chronically have suggested no effect of propafenone on sperm count.
Neuromuscular Dysfunction
Exacerbation of myasthenia gravis has been reported during propafenone therapy.
Drug Interactions
Quinidine: Small doses of quinidine completely inhibit the hydroxylation metabolic pathway, making all patients, in effect, slow metabolizers (see CLINICAL PHARMACOLOGY). There is, as yet, too little information to recommend concomitant use of propafenone and quinidine.
Local Anesthetics: Concomitant use of local anesthetics (i.e., during pacemaker implantations, surgery, or dental use) may increase the risks of central nervous system side effects.
Digitalis: Propafenone hydrochloride produces dose-related increases in serum digoxin levels ranging from about 35% at 450 mg/day to 85% at 900 mg/day of propafenone without affecting digoxin renal clearance. These elevations of digoxin levels were maintained for up to 16 months during concomitant administration. Plasma digoxin levels of patients on concomitant therapy should be measured, and digoxin dosage should ordinarily be reduced when propafenone is started, especially if a relatively large digoxin dose is used or if plasma concentrations are relatively high.
Beta-antagonists: In a study involving healthy subjects, concomitant administration of propafenone and propranolol has resulted in substantial increases in propranolol plasma concentration and elimination half-life with no change in propafenone plasma levels from control values. Similar observations have been reported with metoprolol. Propafenone appears to inhibit the hydroxylation pathway for the two beta-antagonists (just as quinidine inhibits propafenone metabolism). Increased plasma concentrations of metoprolol could overcome its relative cardioselectivity. In propafenone clinical trials, patients who were receiving beta-blockers concurrently did not experience an increased incidence of side effects. While the therapeutic range for beta-blockers is wide, a reduction in dosage may be necessary during concomitant administration with propafenone.
Warfarin: In a study of eight healthy subjects receiving propafenone and warfarin concomitantly, mean steady-state warfarin plasma concentrations increased 39% with a corresponding increase in prothrombin times of approximately 25%. It is therefore recommended that prothrombin times be routinely monitored and the dose of warfarin be adjusted if necessary.
Cimetidine: Concomitant administration of propafenone and cimetidine in 12 healthy subjects resulted in a 20% increase in steady-state plasma concentrations of propafenone with no detectable changes in electrocardiographic parameters beyond that measured on propafenone alone.
Desipramine: Concomitant administration of propafenone and desipramine may result in elevated serum desipramine levels. Both desipramine, a tricyclic antidepressant, and propafenone are cleared by oxidative pathways of demethylation and hydroxylation carried out by the hepatic P-450 cytochrome.
Cyclosporine: Propafenone therapy may increase levels of cyclosporine.
Theophylline: Propafenone may increase theophylline concentration during concomitant therapy with the development of theophylline toxicity.
Rifampin: Rifampin may accelerate the metabolism and decrease the plasma levels and antiarrhythmic efficacy of propafenone.
Other: Limited experience with propafenone combined with calcium antagonists and diuretics has been reported without evidence of clinically significant adverse reactions. Drugs that inhibit CYP2D6, CYP1A2 and CYP3A4 might lead to increased plasma levels of propafenone. When propafenone is administered with inhibitors of these enzymes, the patients should be closely monitored and the dose adjusted accordingly.
Renal and Hepatic Toxicity in Animals
Renal changes have been observed in the rat following 6 months of oral administration of propafenone HCl at doses of 180 and 360 mg/kg/day (about 2 and 4 times, respectively, the maximum recommended human daily dose [MRHD] on a mg/m2 basis). Both inflammatory and non-inflammatory changes in the renal tubules, with accompanying interstitial nephritis, were observed. These changes were reversible, as they were not found in rats allowed to recover for 6 weeks. Fatty degenerative changes of the liver were found in rats following longer durations of administration of propafenone HCl at a dose of 270 mg/kg/day (about 3 times the MRHD on a mg/m2 basis.) There were no renal or hepatic changes at 90 mg/kg/day (equivalent to the MRHD on a mg/m2 basis).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime maximally tolerated oral dose studies in mice (up to 360 mg/kg/day, about twice the maximum recommended human oral daily dose [MRHD] on a mg/m2 basis) and rats (up to 270 mg/kg/day, about 3 times the MRHD on a mg/m2 basis) provided no evidence of a carcinogenic potential for propafenone HCl.
Propafenone HCl tested negative for mutagenicity in the Ames (salmonella) test and the mouse dominant lethal test, and tested negative for clastogenicity in the Chinese hamster micronucleus test, and other in vivo tests for chromosomal aberrations in rat bone marrow and Chinese hamster bone marrow and spermatogonia.
Propafenone HCl, administered intravenously to rabbits, dogs, and monkeys, has been shown to decrease spermatogenesis. These effects were reversible, were not found following oral dosing of propafenone HCl, were seen at lethal or near lethal dose levels and were not seen in rats treated either orally or intravenously (see PRECAUTIONS, Impaired Spermatogenesis). Treatment of male rabbits for 10 weeks prior to mating at an oral dose of 120 mg/kg/day (about 2.4 times the MRHD on a mg/m2 basis) or an intravenous dose of 3.5 mg/kg/day (a spermatogenesis-impairing dose) did not result in evidence of impaired fertility. Nor was there evidence of impaired fertility when propafenone HCl was administered orally to male and female rats at dose levels up to 270 mg/kg/day (about 3 times the MRHD on a mg/m2 basis).
Pregnancy
Teratogenic Effects
Pregnancy Category C: Propafenone HCl has been shown to be embryotoxic (decreased survival) in rabbits and rats when given in oral maternally toxic doses of 150 mg/kg/day (about 3 times the maximum recommended human dose [MRHD] on a mg/m2 basis) and 600 mg/kg/day (about 6 times the MRHD on a mg/m2 basis), respectively. Although maternally tolerated doses (up to 270 mg/kg/day, about 3 times the MRHD on a mg/m2 basis) produced no evidence of embryotoxicity in rats, post-implantation loss was elevated in all rabbit treatment groups (doses as low as 15 mg/kg/day, about 1/3 the MRHD on a mg/m2 basis). There are no adequate and well-controlled studies in pregnant women. Propafenone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
In a study in which female rats received daily oral doses of propafenone HCl from midgestation through weaning of their offspring, doses as low as 90 mg/kg/day (equivalent to the MRHD on a mg/m2 basis) produced increases in maternal deaths. Doses of 360 or more mg/kg/day (4 or more times the MRHD on a mg/m2 basis) resulted in reductions in neonatal survival, body weight gain and physiological development.
Labor and Delivery
It is not known whether the use of propafenone during labor or delivery has immediate or delayed adverse effects on the fetus, or whether it prolongs the duration of labor or increases the need for forceps delivery or other obstetrical intervention.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from propafenone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of propafenone in pediatric patients have not been established.
Geriatric Use
Clinical studies of propafenone hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
PROPAFENONE HCL ADVERSE REACTIONS
Adverse reactions associated with propafenone hydrochloride occur most frequently in the gastrointestinal, cardiovascular, and central nervous systems. About 20% of patients treated with propafenone hydrochloride have discontinued treatment because of adverse reactions.
Adverse reactions reported for >1.5% of 474 SVT patients who received propafenone in U.S. clinical trials are presented in the following table by incidence and percent discontinuation, reported to the nearest percent.
| Incidence | % of Pts. who | |
| (N=480) | Discontinued | |
| Unusual taste | 14% | 1.3% |
| Nausea and/or Vomiting | 11% | 2.9% |
| Dizziness | 9% | 1.7% |
| Constipation | 8% | 0.2% |
| Headache | 6% | 0.8% |
| Fatigue | 6% | 1.5% |
| Blurred Vision | 3% | 0.6% |
| Weakness | 3% | 1.3% |
| Dyspnea | 2% | 1.0% |
| Wide Complex Tachycardia | 2% | 1.9% |
| Congestive Heart Failure | 2% | 0.6% |
| Bradycardia | 2% | 0.2% |
| Palpitations | 2% | 0.2% |
| Tremor | 2% | 0.4% |
| Anorexia | 2% | 0.2% |
| Diarrhea | 2% | 0.4% |
| Ataxia | 2% | 0.0% |
Results of controlled trials in ventricular arrhythmia patients comparing adverse reaction rates on propafenone and placebo, and on propafenone and quinidine are shown in the following table. Adverse reactions reported for ≥1% of the patients receiving propafenone are shown, unless they were more frequent on placebo than propafenone. The most common events were unusual taste, dizziness, first degree AV block, intraventricular conduction delay, nausea and/or vomiting, and constipation. Headache was relatively common also, but was not increased compared to placebo.
| Propafenone/Placebo Trials | Propafenone/Quinidine Trial | |||
| Propafenone | Placebo | Propafenone | Quinidine | |
| (N=247) | (N=111) | (N=53) | (N=52) | |
| Unusual Taste | 7% | 1% | 23% | 0% |
| Dizziness | 7% | 5% | 15% | 10% |
| First Degree AV Block | 5% | 1% | 2% | 0% |
| Headache(s) | 5% | 5% | 2% | 8% |
| Constipation | 4% | 0% | 6% | 2% |
| Intraventricular Conduction Delay | 4% | 0% | – | – |
| Nausea and/or Vomiting | 3% | 1% | 6% | 15% |
| Fatigue | – | – | 4% | 2% |
| Palpitations | 2% | 1% | – | – |
| Blurred Vision | 2% | 1% | 6% | 2% |
| Dry Mouth | 2% | 1% | 6% | 6% |
| Dyspnea | 2% | 3% | 4% | 0% |
| Abdominal Pain/Cramps | – | – | 2% | 8% |
| Dyspepsia | – | – | 2% | 8% |
| Congestive Heart Failure | – | – | 2% | 0% |
| Fever | – | – | 2% | 10% |
| Tinnitus | – | – | 2% | 2% |
| Vision, Abnormal | – | – | 2% | 2% |
| Esophagitis | – | – | 2% | 0% |
| Gastroenteritis | – | – | 2% | 0% |
| Anxiety | 2% | 2% | – | – |
| Anorexia | 2% | 1% | 0% | 2% |
| Proarrhythmia | 1% | 0% | 2% | 0% |
| Flatulence | 1% | 0% | 2% | 0% |
| Angina | 1% | 0% | 2% | 4% |
| Second Degree AV Block | 1% | 0% | – | – |
| Bundle Branch Block | 1% | 0% | 2% | 2% |
| Loss of Balance | 1% | 0% | – | – |
| Diarrhea | 1% | 1% | 6% | 39% |
Adverse reactions reported for ≥1% of 2,127 ventricular arrhythmia patients who received propafenone in U.S. clinical trials are presented in the following table by propafenone daily dose. The most common adverse reactions in controlled clinical trials appeared dose-related (but note that most patients spent more time at the larger doses), especially dizziness, nausea and/or vomiting, unusual taste, constipation, and blurred vision. Some less common reactions may also have been dose-related such as first degree AV block, congestive heart failure, dyspepsia, and weakness. The principal causes of discontinuation were the most common events and are shown in the table.
| % of | |||||
| Incidence by Total Daily Dose | Total Incidence | Pts. Who | |||
| 450 mg | 600 mg | ≥900mg | Discont. | ||
| (N=1430) | (N=1337) | (N=1333) | (N=2127) | ||
| Dizziness | 4% | 7% | 11% | 13% | 2.4% |
| Nausea and/or vomiting | 2% | 6% | 9% | 11% | 3.4% |
| Unusual Taste | 3% | 5% | 6% | 9% | 0.7% |
| Constipation | 2% | 4% | 5% | 7% | 0.5% |
| Fatigue | 2% | 3% | 4% | 6% | 1.0% |
| Dyspnea | 2% | 2% | 4% | 5% | 1.6% |
| Proarrhythmia | 2% | 2% | 3% | 5% | 4.7% |
| Angina | 2% | 2% | 3% | 5% | 0.5% |
| Headache(s) | 2% | 3% | 3% | 5% | 1.0% |
| Blurred Vision | 1% | 2% | 3% | 4% | 0.8% |
| Congestive Heart Failure | 1% | 2% | 3% | 4% | 1.4% |
| Ventricular Tachycardia | 1% | 2% | 3% | 3% | 1.2% |
| Dyspepsia | 1% | 2% | 3% | 3% | 0.9% |
| Palpitations | 1% | 2% | 3% | 3% | 0.5% |
| Rash | 1% | 1% | 2% | 3% | 0.8% |
| AV Block, First Degree | 1% | 1% | 2% | 3% | 0.3% |
| Diarrhea | 1% | 2% | 2% | 3% | 0.6% |
| Weakness | 1% | 2% | 2% | 2% | 0.7% |
| Dry Mouth | 1% | 1% | 1% | 2% | 0.2% |
| Syncope/Near Syncope | 1% | 1% | 1% | 2% | 0.7% |
| QRS Duration, Increased | 1% | 1% | 2% | 2% | 0.5% |
| Chest Pain | 1% | 1% | 1% | 2% | 0.2% |
| Anorexia | 1% | 1% | 2% | 2% | 0.4% |
| Abdominal Pain/Cramps | 1% | 1% | 1% | 2% | 0.4% |
| Ataxia | 0% | 1% | 2% | 2% | 0.2% |
| Insomnia | 0% | 1% | 1% | 2% | 0.3% |
| Premature Ventricular Contraction(s) | 1% | 1% | 1% | 2% | 0.1% |
| Bradycardia | 1% | 1% | 1% | 2% | 0.5% |
| Anxiety | 1% | 1% | 1% | 2% | 0.6% |
| Edema | 1% | 0% | 1% | 1% | 0.2% |
| Tremor(s) | 0% | 1% | 1% | 1% | 0.3% |
| Diaphoresis | 1% | 0% | 1% | 1% | 0.3% |
| Bundle Branch Block | 0% | 1% | 1% | 1% | 0.5% |
| Drowsiness | 1% | 1% | 1% | 1% | 0.2% |
| Atrial Fibrillation | 1% | 1% | 1% | 1% | 0.4% |
| Flatulence | 0% | 1% | 1% | 1% | 0.1% |
| Hypotension | 0% | 1% | 1% | 1% | 0.4% |
| Intraventricuar Conduction Delay | 0% | 1% | 1% | 1% | 0.1% |
| Pain, Joints | 0% | 0% | 1% | 1% | 0.1% |
In addition, the following adverse reactions were reported less frequently than 1% either in clinical trials or in marketing experience (adverse events for marketing experience are given in italics). Causality and relationship to propafenone therapy cannot necessarily be judged from these events.
Cardiovascular System: Atrial flutter, AV dissociation, cardiac arrest, flushing, hot flashes, sick sinus syndrome, sinus pause or arrest, supraventricular tachycardia.
Nervous System: Abnormal dreams, abnormal speech, abnormal vision, apnea, coma, confusion, depression, memory loss, numbness, paresthesias, psychosis/mania, seizures (0.3%), tinnitus, unusual smell sensation, vertigo.
Gastrointestinal: A number of patients with liver abnormalities associated with propafenone therapy have been reported in post-marketing experience. Some appeared due to hepatocellular injury, some were cholestatic and some showed a mixed picture. Some of these reports were simply discovered through clinical chemistries, others because of clinical symptoms including fulminant hepatitis and death. One case was rechallenged with a positive outcome.
Cholestasis (0.1%), elevated liver enzymes (alkaline phosphatase, serum transaminases) [0.2%], gastroenteritis, hepatitis (0.03%).
Hematologic: Agranulocytosis, anemia, bruising, granulocytopenia, increased bleeding time, leukopenia, purpura, thrombocytopenia.
Other: Alopecia, eye irritation, hyponatremia/inappropriate ADH secretion, impotence, increased glucose, kidney failure, positive ANA (0.7%), lupus erythematosis, muscle cramps, muscle weakness, nephrotic syndrome, pain, pruritus.
OVERDOSAGE
The symptoms of overdosage, which are usually most severe within 3 hours of ingestion, may include hypotension, somnolence, bradycardia, intra-atrial and intraventricular conduction disturbances, and rarely convulsions and high grade ventricular arrhythmias. Defibrillation as well as infusion of dopamine and isoproterenol have been effective in controlling rhythm and blood pressure. Convulsions have been alleviated with intravenous diazepam. General supportive measures such as mechanical respiratory assistance and external cardiac massage may be necessary.
PROPAFENONE HCL DOSAGE AND ADMINISTRATION
The dose of Propafenone Hydrochloride Tablets must be individually titrated on the basis of response and tolerance. It is recommended that therapy be initiated with 150 mg propafenone given every eight hours (450 mg/day). Dosage may be increased at a minimum of 3 to 4 day intervals to 225 mg every 8 hours (675 mg/day) and, if necessary, to 300 mg every 8 hours (900 mg/day). The usefulness and safety of dosages exceeding 900 mg per day have not been established. In those patients in whom significant widening of the QRS complex or second or third degree AV block occurs, dose reduction should be considered.
As with other antiarrhythmic agents, in the elderly or in ventricular arrhythmia patients with marked previous myocardial damage, the dose of propafenone hydrochloride tablets should be increased more gradually during the initial phase of treatment.
HOW SUPPLIED
Propafenone Hydrochloride Tablets, 150 mg are available as round, white, film-coated tablets debossed with Watson 582 on one side and bisected on the other side supplied in
Boxes of 10x10 UD 100 NDC 63739-509-10
Propafenone Hydrochloride Tablets, 225 mg are available as round, white, film-coated tablets debossed with Watson 583 on one side and bisected on the other side supplied in
Boxes of 10x10 UD 100 NDC 63739-510-10
Store at 20°-25°C (68°- 77°F). [See USP controlled room temperature.] Dispense in a tight, light-resistant container as defined in the USP.
Manufactured By:
Waston Pharmacueticals
311 Bonnie Circle
Corona, CA 92880
Distributed By:
McKesson Packaging Services
a business unit of McKesson Corporation
7101 Weddington Rd., Concord, NC 28027
IS-4012851
April 2012
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

Propafenone HClPropafenone hydrochloride TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Propafenone HClPropafenone hydrochloride TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||