Propoven
Propoven
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
HEALTH CARE PROVIDER LETTER
|
URGENT: PROPOFOL UPDATE
|
June 19, 2012
Dear Healthcare Professional,
APP® is coordinating with the FDA to increase the availability of propofol products as continuous supply of this essential drug is critical to patient care. Therefore, APP, under an exercise of enforcement discretion by FDA, will reintroduce Fresenius Propoven 1% (propofol 1%) to the US market. Fresenius Propoven 1% (propofol 1%) to be temporarily imported in 2012 is the same anesthesia product that was imported during the 2009 and 2010 propofol drug shortage. Fresenius Propoven 1% (propofol 1%) is manufactured in FDA inspected facilities by APP’s parent company, Fresenius Kabi AG. All of these facilities are in compliance with FDA manufacturing standards.
At this time, FDA has exercised its enforcement discretion to allow for the importation of Fresenius Propoven 1% (propofol 1%) solely by APP during the critical shortage of propofol. Importation or distribution of Fresenius Propoven 1% (propofol 1%) vials by any entity other than APP, or its authorized distributors, is outside the scope of FDA’s enforcement discretion, and Fresenius Propoven 1% (propofol 1%) is not an FDA approved marketed drug in the United States.
Effective immediately, and during this temporary period, APP will offer propofol 1% in the following versions:
|
APP DIPRIVAN
®
|
APP Propofol 1%
(generic DIPRIVAN®) |
Fresenius Propoven 1%
(propofol 1%) |
|
|
20 mL SD Vial |
NDC # 63323-269-20 |
NDC # 63323-270-25 |
N/A |
|
20 mL Ampule |
N/A | N/A |
Product # 831230221 |
|
50 mL SD Vial |
NDC # 63323-269-50 |
NDC # 63323-270-50 |
Product # 4266051 |
|
100 mL SD Vial |
NDC # 63323-269-65 |
NDC # 63323-270-65 |
Product # 4266031 |
APP’s Propofol Injectable Emulsion, USP formulation is identical to DIPRIVAN ®
Fresenius Propoven 1% (propofol 1%) contains the same active ingredient, propofol, in the same concentration as APP DIPRIVAN® (propofol 1%). It is important to note that there are some key differences in the formulation and labeling between the US marketed propofol products and the international Fresenius Propoven 1% (propofol 1%), that you need to be aware of:
- Fresenius Propoven 1% (propofol 1%) contains a combination of long-chain triglycerides (LCT), similar to those found in DIPRIVAN®; however, Fresenius Propoven 1% (propofol 1%) also contains medium-chain triglycerides (MCT) that are not present in the DIPRIVAN® formulation. As with DIPRIVAN® and other propofol products, special care should be applied in patients with disorders of fat metabolism, patients receiving Total Parenteral Nutrition (TPN), and in patients with other conditions where lipid emulsions must be used with caution.
- Fresenius Propoven 1% (propofol 1%) does NOT contain an anti-microbial retardant such as ethylenediaminetetraacetic acid (EDTA), sodium meta-bisulfate, or benzyl alcohol/sodium benzoate.
° STRICT ASEPTIC TECHNIQUE MUST ALWAYS BE MAINTAINED DURING HANDLING.
° Each vial of Fresenius Propoven 1% (propofol 1%) is intended only for single administration for an individual patient. Vials are not intended for multidose use.
° As stated in USP Chapter 797, Pharmaceutical Compounding-Sterile Preparations, the solution content of ampules should be passed through a sterile filter needle to
remove any particles. When withdrawing from Fresenius Propoven 1% (propofol 1%) 20 mL glass ampules, the use of a 5 micron blunt filter needle is
recommended in order to maintain the integrity of the emulsion and reduce the risk of particulate including glass.
° Unused Fresenius Propoven 1% (propofol 1%) should be discarded within 6 hours after being drawn up into a syringe.
° The unused portion of a vial or ampule should be discarded immediately after opening.
° As with any propofol 1% used for IV infusion, discard all product and infusion therapy systems after 12 hours.
- Fresenius Propoven 1% (propofol 1%) is contraindicated in patients who are allergic to soy or peanut.
- The barcode used on Fresenius Propoven 1% (propofol 1%) product is an international pharmaceutical manufacturing code and may not be appropriately recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is scanned.
- Alternative procedures should be followed to assure that the correct drug product is being prepared and administered to individual patients.
- Fresenius Propoven 1% (propofol 1%) product information sheet contains a patient information leaflet as part of the international requirement for propofol 1%. For questions regarding Fresenius Propoven 1% (propofol 1%) in the United States, please contact APP Vigilance and Medical Affairs at 1-800-551-7176, Monday - Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail appmedicalinfo@APPpharma.com.
- The attached product comparison table highlights the key differences in formulation and labeling between APP DIPRIVAN® and Fresenius Propoven 1% (propofol 1%).
| Please find attached a copy of the Fresenius Propoven 1% package insert. |
To further supplement supply, APP maintains its commitment to expedite the manufacturing of DIPRIVAN® and Propofol to maintain continuous releases on all product presentations. We believe the combination of continuous APP DIPRIVAN® and APP Propofol releases in addition to availability of Fresenius Propoven 1% (propofol 1%) will help alleviate a market shortage.
Please evaluate the use of Fresenius Propoven (propofol 1%) in your institution and begin placing orders immediately. If your institution desires not to use Fresenius Propoven (propofol 1%), you may continue to order APP DIPRIVAN® and/or APP Propofol; APP will reserve some DIPRIVAN® and/or Propofol for those patients where Fresenius Propoven (propofol 1%) is contraindicated.
All direct customer orders for propofol products, including APP DIPRIVAN®, APP Propofol, and Fresenius Propoven (propofol 1%), will remain on an allocation process. APP will closely monitor the distribution of all propofol products to help manage imbalances in supply. APP will continue to replenish the distribution channel with all propofol products, and encourage customers to check with wholesaler and distributor partners for product availability of APP DIPRIVAN®, APP Propofol, and Fresenius Propoven (propofol 1%). Please be aware that Fresenius Propoven (propofol 1%) is not returnable and not for resale.
If you have additional questions, please contact Customer Service at 1-888-386-1300, Monday - Friday, between the hours of 7 a.m. and 6 p.m. (CST) or APP Vigilance and Medical Affairs at 1-800-551-7176, Monday - Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail appmedicalinfo@APPpharma.com. This communication and updated product information is available on the APP web site www.APPpharma.com as well as on the FDA Drug Shortage web site http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm.
To report adverse events among patients administered Fresenius Propoven 1% (propofol 1%), please call 1-800-551-7176, Monday - Friday, between the hours of 8 a.m. and 5 p.m. (CST). Adverse event information may also be reported to FDA’s MedWatch Adverse Reporting System either online, by regular mail or by fax:
- Online: www.fda.gov/medwatch/report.htm
- Regular Mail: use postage-paid FDA form 3500 available at: www.fda.gov/MedWatch/getforms.htm
Mail to MedWatch, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787
- Fax: 1-800-FDA-0178
If you have any questions about the information contained in this letter or the safe and effective use of Fresenius Propoven 1% (propofol 1%), please contact our Vigilance and Medical Affairs department at 1-800-551-7176, Monday - Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail appmedicalinfo@APPpharma.com
Sincerely,
Mitchell L. Ehrlich
Vice President, Quality
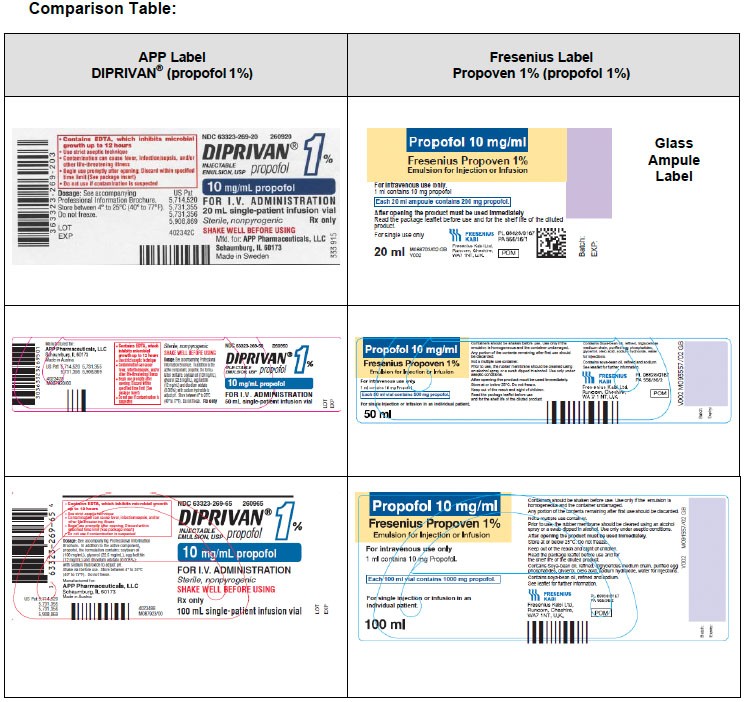
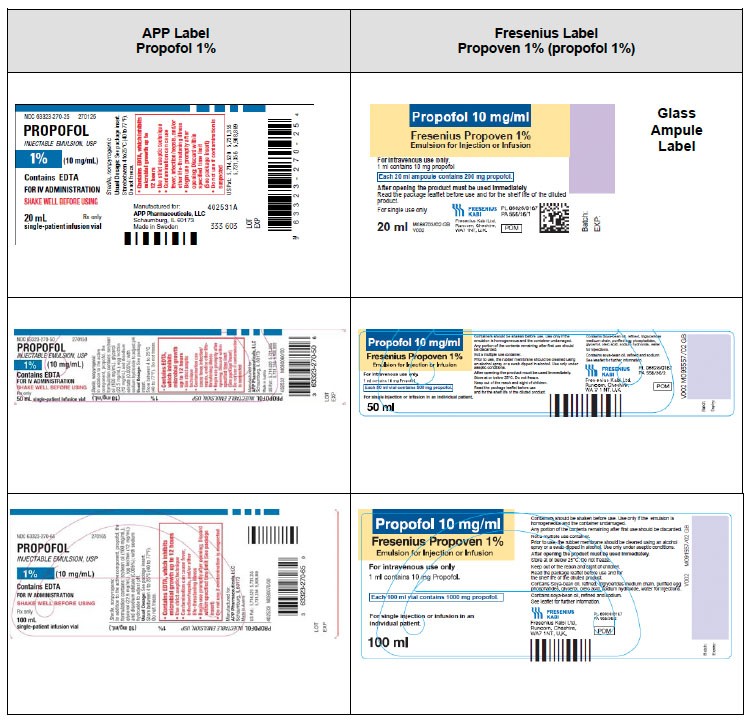
|
APP DIPRIVAN ® and APP Propofol (propofol 1%) |
Fresenius Propoven 1% (propofol 1%) |
What does this mean to you, as a Healthcare Professional? |
|
Contains ethylenediaminetetraacetic acid (EDTA) |
Does not contain a preservative such as ethylenediaminetetraacetic acid (EDTA), benzyl alcohol/sodium benzoate, or sodium meta-bisulfate
|
Fresenius Propoven 1% (propofol 1%) is a single dose vial for administration to a single patient. Strict aseptic technique must always be maintained during handling
|
|
Indications and contraindications: see package insert |
Indications and contraindications: see package insert
Please note: see package insert on 4.2 method of administration, 4.3 contraindications, and 4.4 special warning and precautions for use |
Fresenius Propoven 1% (propofol 1%) may be used for induction of anesthesia in patients above the age of 3 years or for maintenance of anesthesia in patients above the age of 2 months. Fresenius Propoven 1% (propofol 1%) is contraindicated in patients who are allergic to soy or peanut. |
|
Contains long-chain triglycerides (LCT)
|
Contains a combination of medium-chain triglycerides (MCT) plus long-chain triglycerides (LCT)
|
The presence of MCTs should be taken into consideration when treating patients with disorders of fat metabolism or who are receiving TPN.
|
|
Unit of use barcode on individual vials
|
No unit of use barcode
|
The barcode used on Fresenius Propoven 1% (propofol 1%) may not register accurately on US scanning systems. Other means of confirming the correct drug is being prepared and administered to the correct patient should be utilized.
|
|
N/A |
Contains a patient information leaflet .
|
For questions regarding Fresenius Propoven 1% (propofol 1%) in the United States, please contact APP Vigilance and Medical Affairs at 1-800-551-7176, Monday – Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail appmedicalinfo@APPpharma.com .
|
|
Only available in Single Dose Vials (SDV) |
Available in 20 mL glass ampule and 50 mL & 100 mL single dose vials (SDV)
|
When withdrawing from Fresenius Propoven 1% (propofol 1%) 20 mL glass ampules, the use of a 5 micron blunt filter needle is recommended in order to maintain the integrity of the emulsion and reduce the risk of particulate including glass.
|
PACKAGE LEAFLET: INFORMATION FOR THE USER
Fresenius Propoven 1%
Emulsion for Injection or Infusion
Propofol
|
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. - If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. |
- What Fresenius Propoven 1% is and what it is used for
- Before you use Fresenius Propoven 1%
- How to use Fresenius Propoven 1%
- Possible side effects
- How to store Fresenius Propoven 1%
- Further information
1. WHAT FRESENIUS PROPOVEN 1% IS AND WHAT IT IS USED FOR
Fresenius Propoven 1% belongs to a group of medicines called general anaesthetics. General anaesthetics are used to cause unconsciousness (sleep) so that surgical operations or other procedures can be performed. They can also be used to sedate you (so that you are sleepy but not completely asleep).
Propofol is used to:
- induce and maintain general anaesthesia in adults and children > 1 month.
- sedate patients > 16 years of age receiving artificial respiration in intensive care.
- sedate adults and children > 1 month during diagnostic and surgical procedures, alone or in combination with local or regional anaesthesia
2. BEFORE YOU USE FRESENIUS PROPOVEN 1%
DO NOT use Fresenius Propoven 1%
- if you are hypersensitive (allergic) to propofol or to any of the other ingredients of this medicine (see section 6 “Further information” at the end of this leaflet).
- if you are hypersensitive (allergic) to soya or peanut (see “Important information about some of the ingredients of Fresenius Propoven 1% at the end of section 2).
- in patients of 16 years of age or younger for sedation in intensive care.
Take special care with Fresenius Propoven 1%
You should not receive Fresenius Propoven 1%, or only under extreme caution and intensive monitoring, if you:
- have advanced heart failure
- have any other serious disease of the heart
- are receiving electroconvulsive therapy (ECT, a treatment for psychiatric problems)
The use of Fresenius Propoven 1% is not recommended in newborn infants. Special care should also be observed when administering Fresenius Propoven 1% to children less than 3 years of age. However, evidence now available does not suggest that this is any less safe than in older children. The safety of propofol for sedation in chidren and adolescents 16 years of age and younger in the intensive care unit has not been demonstrated.
In general, Fresenius Propoven 1% should be given with caution to elderly or weak patients.
Before receiving Fresenius Propoven 1%, tell your anaesthetist or intensive care doctor if you have:
- heart disease
- lung disease
- kidney disease
- liver disease
- seizures (epilepsy)
- a raised pressure inside the skull (raised intracranial pressure). In combination with low blood pressure the amount of blood reaching the brain may be decreased.
- altered levels of fat in the blood. If you are receiving total parenteral nutrition (feeding through a vein), the levels of fat in your blood must be monitored.
If you have any of the following conditions, they must be treated before you receive Fresenius Propoven 1%:
- heart failure
- when there is insufficient blood reaching the tissues (circulatory failure)
- severe breathing problems (respiratory failure)
- dehydration (hypovolaemia)
- seizures (epilepsy)
Fresenius Propoven 1% may increase the risk of
- epileptic seizures
- a nervous reflex that slows the heart rate (vagotonia, bradycardia)
- changes in the blood flow to the organs of the body (haemodynamic effects on the cardiovascular system) if you are overweight and receive high doses of Fresenius Propoven 1%.
Involuntary movements can occur during sedation with Fresenius Propoven 1%. The doctors will take into account how this might affect surgical procedures being performed under sedation and will take the necessary precautions.
Very occasionally, after anaesthesia, there may be a period of unconsciousness associated with stiffness of the muscles. This requires observation by the medical staff but no other treatment. It will resolve spontaneously.
The injection of Fresenius Propoven 1% can be painful. A local anaesthetic can be used to reduce this pain but can have its own side effects.
You will not be allowed to leave the hospital until you are fully awake.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
You must take special care if you are also taking any of the following medicines:
- premedications (your anaesthetist will know which medicines may interact with Fresenius Propoven 1%)
- other anaesthetics, including general, regional, local and inhalational anaesthetics (lower doses of Fresenius Propoven 1% may be required. Your anaesthetist will know this)
- analgesics (painkillers)
- drugs that relax muscles, e.g. suxamethonium
- benzodiazepines (drugs for anxiety)
- drugs that affect many of the internal body functions such as the heart rate, e.g. atropine
- strong painkillers, e.g. fentanyl
- alcohol
- neostigmine (a treatment for muscle weakness)
- cyclosporin (used to prevent transplant rejections)
Using Fresenius Propoven 1% with food and drink
After you have been given Fresenius Propoven 1%, you should not drink alcohol until fully recovered.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Fresenius Propoven 1% should not be given to pregnant women unless necessary. Mothers should stop breast-feeding and discard any breast milk for 24 hours after receiving Fresenius Propoven 1%.
Driving and using machines
After you have been given Fresenius Propoven 1%, you must not drive, operate machinery, or work in dangerous situations. You should not go home alone.
Important information about some of the ingredients of Fresenius Propoven 1%
Fresenius Propoven 1% contains soya-bean oil. This can rarely cause severe hypersensitivity (allergic) reactions (see “Do not use Fresenius Propoven 1%”). Tell your doctor if you know that you have allergic reactions to soya-bean oil.
This medicinal product contains less than 1 mmol (23 mg) sodium per 100 ml, i.e. essentially ´sodium-free`.
3. HOW TO USE FRESENIUS PROPOVEN 1%
Fresenius Propoven 1% will only be given to you in hospitals or suitable therapy units by your anaesthetist or by an intensive care doctor.
The dose you are given will vary depending on your age, body weight and physical condition. The doctor will give the correct dose to start and to sustain anaesthesia or to achieve the required level of sedation, by carefully watching your responses and vital signs (pulse, blood pressure, breathing etc.). It can also be affected by other medicines you may be taking. Most people need 1.5 to 2.5 mg propofol per kg body weight to make them go to sleep (induction of anaesthesia), and then 4 to 12 mg propofol per kg body weight per hour after this to keep them asleep (maintenance of anaesthesia). For sedation, doses of 0.3 to 4 mg propofol per kg body weight per hour are usually sufficient.
For sedation during surgical and diagnostic procedures in adults, most patients will require 0.5 to 1 mg propofol per kg body weight over 1 to 5 minutes for onset of sedation. Maintenance of sedation may be accomplished by titrating Fresenius Propoven 1% infusion to the desired level of sedation. Most patients will require 1.5 to 4.5 mg propofol per kg body weight per hour. The infusion may be supplemented by bolus administration of 10 to 20 mg propofol (1 to 2 ml Fresenius Propoven 1% if a rapid increase of the depth of sedation is required.
Fresenius Propoven 1% is for intravenous use, usually on the back of the hand or in the forearm. Your anaesthetist may use a needle or cannula (a fine plastic tube). An electric pump may be used to give the injection for long operations and for use in intensive care.
Elderly and weak patients may require lower doses.
Children usually require slightly higher doses. The dose should be adjusted according to age and/or body weight.
When used for sedation, Fresenius Propoven 1% must not be administered for more than 7 days.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Fresenius Propoven 1% can cause side effects, although not everybody gets them.
Evaluation of the side effects is based on the following frequencies:
|
Very Common |
affects more than 1 user in 10 |
|
Common |
affects 1 to 10 users in 100 |
|
Uncommon |
affects 1 to 10 users in 1,000 |
|
Rare |
affects 1 to 10 users in 10,000 |
|
Very rare |
affects less than 1 user in 10,000 |
|
Not known |
frequency cannot be estimated from the available data |
If you think you have any of the below mentioned side effects or any other side effects, please inform a physician as soon as possible.
Very common:
- local pain during the injection.
Common:
- increase of levels of fat in the blood (hypertriglyceridemia)
These side effects may occur during the induction of anaesthesia:
- muscle jerks (myoclonus)
- muscle twitching (minimal excitation)
- low blood pressure (hypotension)
- slow heartbeat (bradycardia)
- rapid heartbeat (tachycardia)
- hot flushes
- increased breathing (hyperventilation)
- stopping breathing (temporary apnoea)
- coughing after anaesthesia
- hiccups (singultus)
Uncommon:
- severe low blood pressure (hypotension)
- coughing during anaesthesia
- slowing of the pulse rate (progressive
bradycardia)
Rare:
- a severe allergic reaction , including:
- swelling of the skin of the face, mouth and throat (angioedema)
- narrowing of the airways in the lungs that makes it difficult to breathe (bronchospasm)
- reddening of the skin (erythema)
- low blood pressure (hypotension)
- headache
- dizziness (vertigo)
- epileptiform movements (involuntary movements similar to epilepsy), including convulsions and opisthotonus ( a rigid posture with the head arched backwards)
- blood clots (thrombosis)
- inflammation of the blood vessels (phlebitis)
- discoloration of urine
- postoperative fever
These rare side effects may occur during the recovery period (waking up):
- euphoria (feeling happy) and sexual arousal
- shivering and feeling cold
- irregular heartbeat (arrhythmia)
- coughing
- feeling sick (nausea) or vomiting
Very rare:
- allergic reactions caused by soya-bean oil
- delayed epileptiform attacks (involuntary movements similar to epilepsy after waking up)
- convulsions in epileptic patients
- unconsciousness after anaesthesia
- fluid on the lungs (pulmonary oedema)
- inflammation of the pancreas (pancreatitis)
- severe tissue responses after accidental injection into tissues
- rhabdomyolysis (a disorder of the muscle)
- a change in the acidity of the blood (metabolic acidosis)
- a high level of potassium in the blood (hyperkalaemia)
- heart failure.
When Fresenius Propoven 1% is administered in combination with lidocaine (a local anaesthetic used to reduce the pain at the site of injection), certain side effects may occur rarely:
- dizziness
- vomiting
- sleepiness
- fits
- a slowing of the heart rate (bradycardia)
- irregular heartbeat (cardiac arrhythmias)
- shock
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE FRESENIUS PROPOVEN 1%
Keep out of the reach and sight of children.
Do not use Fresenius Propoven 1% after the expiry date which is stated on the ampoule/vial and the outer packaging after EXP. The expiry date refers to the last day of that month.
Store at or below 25°C.
Do not freeze.
After opening the product must be used immediately. Administration systems with undiluted Fresenius Propoven 1% should be replaced 12 hours after opening of the ampoule or vial. Dilutions with 5% w/v glucose solution or 0.9% w/v sodium chloride intravenous infusion solution or an admixture 1% preservative-free lidocaine injection solution (at least 2 mg propofol per ml) should be prepared aseptically (controlled and validated conditions preserved) immediately before administration and has to be administered within 6 hours after preparation.
Containers should be shaken before use. If two layers can be seen after shaking, the emulsion should not be used. Use only homogenous preparations and undamaged containers.
For single use. Any unused emulsion must be discarded.
Medicines should not be disposed via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. FURTHER INFORMATION
What Fresenius Propoven 1% contains
- The active substance is propofol.
1 ml emulsion contains 10 mg propofol.
Each 20 ml ampoule contains 200 mg propofol.
Each 20 ml vial contains 200 mg propofol.
Each 50 ml vial contains 500 mg propofol.
Each 100 ml vial contains 1000 mg propofol.
- The other ingredients are soya-bean oil refined, triglycerides medium-chain, purified egg phosphatides, glycerol, oleic acid, sodium hydroxide, water for injections
What Fresenius Propoven 1% looks like and contents of the pack
Fresenius Propoven 1% is a white oil-in-water emulsion for injection or infusion.
Fresenius Propoven 1% is available in colourless glass ampoules or glass vials. The glass vials are sealed with rubber stoppers.
Pack sizes:
Packs containing 5 glass ampoules with 20 ml emulsion
Packs containing 1 glass vial with 20, 50 or 100 ml emulsion
Packs containing 5 glass vials with 20 ml emulsion
Packs containing 10 glass vials with 20, 50 or 100 ml emulsion
Packs containing 15 glass vials with 50 or 100 ml emulsion
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Fresenius Kabi Ltd
Cestrian Court, Eastgate Way
Manor Park, Runcorn
Cheshire, WA7 1NT
UK
Manufacturer:
Fresenius Kabi Austria GmbH
A-8055 Graz, Hafnerstrasse 36
Austria
Fresenius Kabi AB
S-75174 Uppsala, Rapsgatan 7
Sweden
This leaflet was last approved in 10/2010
This medicinal product is authorised in the Member States of the EEA under the following names:
Country Trade Name
Austria Propofol “Fresenius” 1 % mit
MCT - Emulsion zur Injektion oder Infusion
Belgium Propolipid 1 %
Cyprus Propofol 1% MCT/LCT Fresenius
Czech Republic Propofol 1% MCT/LCT Fresenius
Denmark Propolipid
Estonia Propoven 1%
Germany Propofol 1% (10 mg/1 ml) MCT
Fresenius, Emulsion zur Injektion oder Infusion
Greece Propofol MCT/LCT 1%
Finland Propolipid 10 mg/ml
Hungary Propofol 1% MCT/LCT Fresenius
Iceland Propolipid 10 mg/ml
Ireland Fresenius Propoven 1%
Italy Propofol Kabi 10mg/ml
Latvia Propoven 1%
Lithuania Propoven 1%
Luxembourg Propofol 1% MCT Fresenius
Netherlands Propofol 10mg/ml MCT/LCT
Fresenius
Norway Propolipid 10 mg/ml
Poland Propofol 1% MCT/LCT Fresenius
Portugal Propofol 1% MCT/LCT Fresenius
Slovakia Propofol 1% MCT/LCT Fresenius
Slovenia Propoven 10 mg/ml emulzija za injiciranje ali infundiranje
Spain Propofol Lipomed 10 mg/ml
Fresenius emulsión para inyección o perfusión
Sweden Propolipid 10 mg/ml
United Kingdom Fresenius Propoven 1%
The following information is intended for medical or healthcare professionals only:
Fresenius Propoven 1% should not be mixed prior to administration with injection or infusion solutions other than 5% w/v glucose solution or 0.9% w/v sodium chloride intravenous infusion solution or 1% preservative-free lidocaine injection solution. Final propofol concentration must not be below 2 mg/ml.
For single use. Any unused emulsion must be discarded.
Containers should be shaken before use.
If two layers can be seen after shaking, the emulsion should not be used.
Use only homogeneous preparations and undamaged containers.
Prior to use, the ampoule neck or rubber membrane should be cleaned using an alcohol spray or a swab dipped in alcohol. After use, tapped containers must be discarded.
Fresenius Propoven 1% must only be given in hospitals or adequately equipped therapy units by physicians trained in anaesthesia or in intensive care. For sedation during surgical and diagnostic procedures Fresenius Propoven 1% should not be administered by the same person conducting the surgical or diagnostic procedure.
Circulatory and respiratory functions should be constantly monitored (e.g. ECG, pulse oxymetry) and facilities for maintenance of patient airways, artificial ventilation, and other resuscitation facilities should be immediately available at all times.
Propofol may be administered undiluted or diluted in 5% w/v glucose or 0.9% w/v sodium chloride intravenous infusion solutions.
5% w/v glucose intravenous infusion solution, 0.9% w/v sodium chloride intravenous solution or 0.18% w/v sodium sodium chloride and 4% w/v glucose intravenous infusion solution may be given through the same infusion set. Fresenius Propoven 1% must not be mixed with any other solutions for infusion or injection.
Co-administration of other medicinal products or fluids added to the Fresenius Propoven 1% infusion line must occur close to the cannula site using a Y-piece connector or a three-way valve.
Fresenius Propoven 1% is a lipid containing emulsion without antimicrobial preservatives and may support rapid growth of microorganisms.
The emulsion must be drawn aseptically into a sterile syringe or giving set immediately after opening the ampoule or breaking the vial seal. Administration must commence without delay.
Asepsis must be maintained for both Fresenius Propoven 1% and the infusion equipment throughout the infusion period. Fresenius Propoven 1% must not be administered through a microbiological filter.
I nfusion of undiluted Fresenius Propoven 1%:
The use of a burette, drop counter, syringe pump or volumetric infusion pump to control the infusion rate is recommended when Fresenius Propoven 1% is infused undiluted.
As usual for fat emulsions, the infusion of Fresenius Propoven 1% via one infusion system must not exceed 12 hours. The infusion set for Fresenius Propoven 1% must be changed at least every 12 hours.
Infusion of diluted Fresenius Propoven 1%:
Burettes, drop counters or volumetric infusion pumps should always be used to control infusion rates. The maximum dilution must not exceed 1 part of Fresenius Propoven 1% to 4 parts of 5% w/v glucose or 0.9% w/v sodium chloride intravenous infusion solution (minimum concentration 2 mg propofol per ml). The mixture should be prepared aseptically and administered within 6 hours.
If the same injection system used for the Fresenius Propoven 1% is to be used for the injection of muscle relaxants (e.g. atracurium and mivacurium), the injection system must first be flushed.
Lidocaine may be added to the diluted solution (20 parts of Fresenius Propoven 1% to 1 part of 1% preservative-free lidocaine solution for injection) to reduce pain at the site of injection of Fresenius Propoven 1%. Lidocaine must not be used in patients with hereditary acute porphyria. Muscle relaxants like atracurium and mivacurium should only be administered after flush of the same infusion site used for Fresenius Propoven 1%.
M088702/02 GB
V001
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Propofol 100 mL Vial Carton Panel
Propofol
10 mg/ml
Fresenius Propoven 1%
Emulsion for Injection or Infusion
For intravenous use only

PropovenPROPOFOL INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||