Propranolol Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- PROPRANOLOL HYDROCHLORIDE DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- DRUG INTERACTIONS
- PHARMACODYNAMICS
- INDICATIONS & USAGE
- PROPRANOLOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PROPRANOLOL HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PROPRANOLOL HYDROCHLORIDE DESCRIPTION
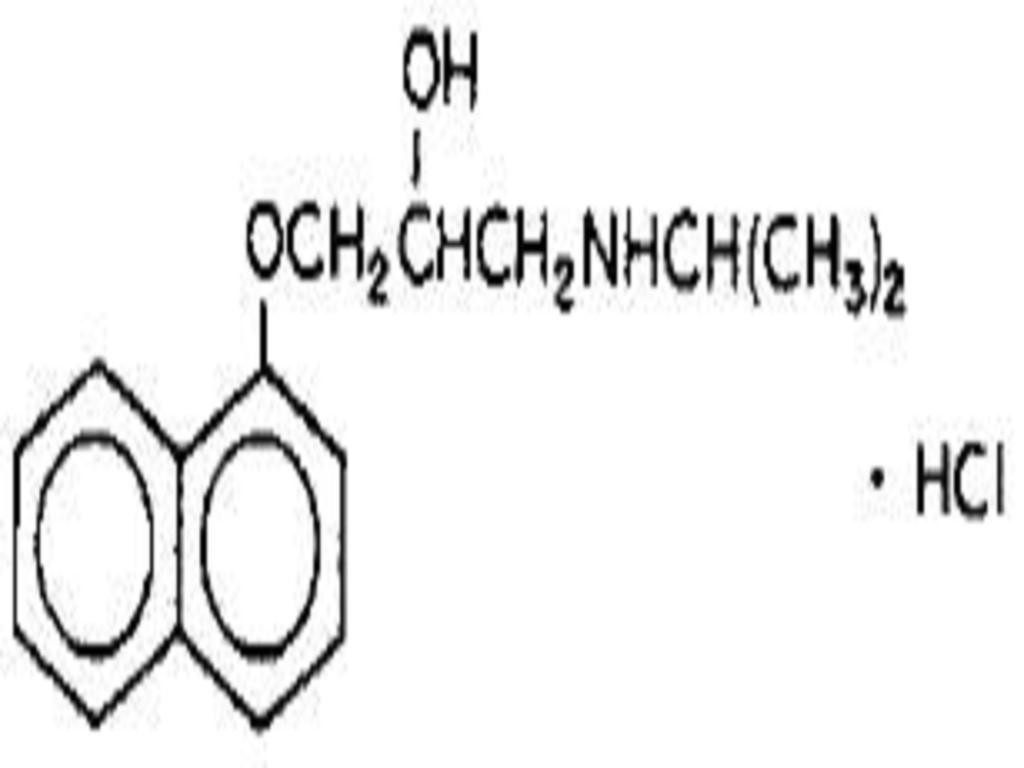
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
GeneralMechanism of Action
PHARMACOKINETICS
AbsorptionDistribution
Metabolism and Elimination
Enantiomers
Special Populations
PRECAUTIONS
DRUG INTERACTIONS
Drug Interactions under PRECAUTIONS
PHARMACODYNAMICS
HypertensionAngina Pectoris
Atrial Fibrillation
Myocardial Infarction
Migraine
Essential Tremor
Hypertrophic Subaortic Stenosis
Pheochromocytoma
INDICATIONS & USAGE
HypertensionAngina Pectoris Due to Coronary Atherosclerosis
Atrial Fibrillation
Myocardial Infarction
Migraine
Essential Tremor
Hypertrophic Subaortic Stenosis
Pheochromocytoma
PROPRANOLOL HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Angina PectorisHypersensitivity and Skin Reactions
ADVERSE REACTIONS
ADVERSE REACTIONS
Cardiac Failure
Nonallergic Bronchospasm (e.g., Chronic Bronchitis, Emphysema)
Major Surgery
Diabetes and Hypoglycemia
Thyrotoxicosis
Wolff-Parkinson-White Syndrome
Pheochromocytoma
PRECAUTIONS
GeneralClinical Laboratory Tests
DRUG INTERACTIONS
Drug Interactions in PHARMACOKINETICS AND DRUG METABOLISMOVERDOSAGE
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Pregnancy Category CNURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
PROPRANOLOL HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
GeneralHypertension
Angina Pectoris
WARNINGS
Atrial Fibrillation
Myocardial Infarction
PHARMACODYNAMICS AND CLINICAL EFFECTS
Migraine
Essential Tremor
Hypertrophic Subaortic Stenosis
Pheochromocytoma
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Propranolol HydrochloridePropranolol Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!