Propylthiouracil
Cardinal Health
Actavis Elizabeth LLC
PROPYLTHIOURACIL TABLETS, USP 40-9158
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
WARNING: Severe liver injury and acute liver failure, in some cases fatal, have been reported in patients treated with propylthiouracil. These reports of hepatic reactions include cases requiring liver transplantation in adult and pediatric patients.
Propylthiouracil should be reserved for patients who can not tolerate methimazole and in whom radioactive iodine therapy or surgery are not appropriate treatments for the management of hyperthyroidism.
Because of the risk of fetal abnormalities associated with methimazole, propylthiouracil may be the treatment of choice when an antithyroid drug is indicated during or just prior to the first trimester of pregnancy (see Warnings and Precautions).
Propylthiouracil, USP is one of the thiocarbamide compounds. It is a white, crystalline substance that has a bitter taste and is very slightly soluble in water. Propylthiouracil is an antithyroid drug administered orally. The structural formula is:
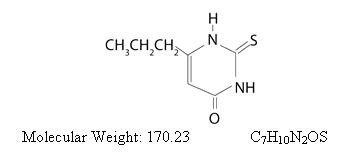
Each tablet contains propylthiouracil, USP 50 mg and the following inactive ingredients: lactose monohydrate, corn starch, colloidal silicon dioxide, povidone, pregelatinized starch, and magnesium stearate.
Propylthiouracil inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and triiodothyronine that are stored in the thyroid or circulating in the blood, nor does it interfere with the effectiveness of thyroid hormones given by mouth or by injection. Propylthiouracil inhibits the conversion of thyroxine to triiodothyronine in peripheral tissues and may therefore be an effective treatment for thyroid storm.
Propylthiouracil is readily absorbed and is extensively metabolized. Approximately 35% of the drug is excreted in the urine, in intact and conjugated forms, within 24 hours.
Propylthiouracil tablets are indicated:
- in patients with Graves’ disease with hyperthyroidism or toxic multinodular goiter who are intolerant of methimazole and for whom surgery or radioactive iodine therapy is not an appropriate treatment option
- to ameliorate symptoms of hyperthyroidism in preparation for thyroidectomy or radioactive iodine therapy in patients who are intolerant of methimazole
Propylthiouracil is contraindicated in patients who have demonstrated hypersensitivity to the drug or any of the other product components.
Liver Toxicity
Liver injury resulting in liver failure, liver transplantation, or death, has been reported with propylthiouracil therapy in adult and pediatric patients. No cases of liver failure have been reported with the use of methimazole in pediatric patients. For this reason, propylthiouracil is not recommended for pediatric patients except when methimazole is not well-tolerated and surgery or radioactive iodine therapy are not appropriate therapies.
There are cases of liver injury, including liver failure and death, in women treated with propylthiouracil during pregnancy. Two reports of in utero exposure with liver failure and death of a newborn have been reported. The use of an alternative antithyroid medication (e.g., methimazole) may be advisable following the first trimester of pregnancy (see PRECAUTIONS, Pregnancy).
Biochemical monitoring of liver function (bilirubin, alkaline phosphatase) and hepatocellular integrity (ALT, AST) is not expected to attenuate the risk of severe liver injury due to its rapid and unpredictable onset. Patients should be informed of the risk of liver failure. Patients should be instructed to report any symptoms of hepatic dysfunction (anorexia, pruritus, right upper quadrant pain, etc.), particularly in the first six months of therapy. When these symptoms occur, propylthiouracil should be discontinued immediately and liver function tests and ALT and AST levels obtained.
Agranulocytosis
Agranulocytosis occurs in approximately 0.2% to 0.5% of patients and is a potentially life-threatening side effect of propylthiouracil therapy. Agranulocytosis typically occurs within the first 3 months of therapy. Patients should be instructed to immediately report any symptoms suggestive of agranulocytosis, such as fever or sore throat. Leukopenia, thrombocytopenia, and aplastic anemia (pancytopenia) may also occur. Propylthiouracil should be discontinued if agranulocytosis, aplastic anemia (pancytopenia), ANCA-positive vasculitis, hepatitis, interstitial pneumonitis, fever, or exfoliative dermatitis is suspected, and the patient's bone marrow indices should be obtained.
Hypothyroidism
Propylthiouracil can cause hypothyroidism necessitating routine monitoring of TSH and free T4 levels with adjustments in dosing to maintain a euthyroid state. Because the drug readily crosses placental membranes, propylthiouracil can cause fetal goiter and cretinism when administered to a pregnant woman (see PRECAUTIONS, Pregnancy).
Patients should be instructed to report any symptoms of hepatic dysfunction (anorexia, pruritus, jaundice, light colored stools, dark urine, right upper quadrant pain, etc.), particularly in the first six months of therapy. When these symptoms occur, measurement should be made of liver function (bilirubin, alkaline phosphatase) and hepatocellular integrity (ALT/AST levels).
Patients who receive propylthiouracil should be under close surveillance and should be counseled regarding the necessity of immediately reporting any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. In such cases, white blood cell and differential counts should be obtained to determine whether agranulocytosis has developed. Particular care should be exercised with patients who are receiving concomitant drugs known to be associated with agranulocytosis.
Patients should be advised that if they become pregnant or intend to become pregnant while taking an antithyroid drug, they should contact their physician immediately about their therapy.
Patients should report immediately any evidence of illness, in particular sore throat, skin eruptions, fever, headache, or general malaise. They also should report symptoms suggestive of hepatic dysfunction (anorexia, pruritus, right upper quadrant pain, etc.).
Because propylthiouracil may cause hypoprothrombinemia and bleeding, monitoring of prothrombin time should be considered during therapy with the drug, especially before surgical procedures.
Thyroid function tests should be monitored periodically during therapy. Once clinical evidence of hyperthyroidism has resolved, the finding of an elevated serum TSH indicates that a lower maintenance dose of propylthiouracil should be employed.
Anticoagulants (Oral): Due to the potential inhibition of vitamin K activity by propylthiouracil, the activity of oral anticoagulants (e.g., warfarin) may be increased; additional monitoring of PT/INR should be considered, especially before surgical procedures.
Beta-adrenergic Blocking Agents: Hyperthyroidism may cause an increased clearance of beta blockers with a high extraction ratio. A reduced dose of beta-adrenergic blockers may be needed when a hyperthyroid patient becomes euthyroid.
Digitalis Glycosides: Serum digitalis levels may be increased when hyperthyroid patients on a stable digitalis glycoside regimen become euthyroid; a reduced dose of digitalis glycosides may be needed.
Theophylline: Theophylline clearance may decrease when hyperthyroid patients on a stable theophylline regimen become euthyroid; a reduced dose of theophylline may be needed.
Laboratory animals treated with propylthiouracil for > 1 year have demonstrated thyroid hyperplasia and carcinoma formation. Such animal findings are seen with continuous suppression of thyroid function by sufficient doses of a variety of antithyroid agents, as well as in dietary iodine deficiency, subtotal thyroidectomy, and implantation of autonomous thyrotropic hormone-secreting pituitary tumors. Pituitary adenomas have also been described.
Because propylthiouracil readily crosses placental membranes and can induce goiter and even cretinism in the developing fetus, it is important that a sufficient, but not excessive, dose be given during pregnancy. In many pregnant women, the thyroid dysfunction diminishes as the pregnancy proceeds; consequently a reduction of dosage may be possible. In some instances, propylthiouracil can be withdrawn several weeks or months before delivery.
If propylthiouracil is used during pregnancy, or if the patient becomes pregnant while taking propylthiouracil, the patient should be warned of the rare potential hazard to the mother and fetus of liver damage.
Since methimazole may be associated with the rare development of fetal abnormalities such as aplasia cutis and choanal atresia, propylthiouracil may be the preferred agent during organogenesis, in the first trimester of pregnancy. Given the potential maternal adverse effects of propylthiouracil (e.g., hepatotoxicity), it may be preferable to switch from propylthiouracil to methimazole for the second and third trimesters.
Pregnancy Category D: See WARNINGS.
Propylthiouracil is transferred to breast milk to a small extent and therefore likely results in clinically insignificant doses to the suckling infant. In one study, nine lactating women were administered 400 mg of propylthiouracil by mouth. The mean amount of propylthiouracil excreted during 4 hours after drug administration was 0.025% of the administered dose.
Postmarketing reports of severe liver injury including hepatic failure requiring liver transplantation or resulting in death have been reported in the pediatric population. No such reports have been observed with methimazole. As such, propylthiouracil is not recommended for use in the pediatric population except in rare instances in which methimazole is not well-tolerated and surgery or radioactive iodine therapy are not appropriate.
When used in children, parents and patients should be informed of the risk of liver failure. If patients taking propylthiouracil develop tiredness, nausea, anorexia, fever, pharyngitis, or malaise, propylthiouracil should be discontinued immediately by the patient, a physician should be contacted, and a white blood cell count, liver function tests, and transaminase levels obtained.
Clinical studies of propylthiouracil did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Major adverse reactions (much less common than the minor adverse reactions) include liver injury resulting in hepatitis, liver failure, a need for liver transplantation or death. Inhibition of myelopoiesis (agranulocytosis, granulopenia, and thrombocytopenia), aplastic anemia, drug fever, a lupus-like syndrome (including splenomegaly and vasculitis), hepatitis, periarteritis, and hypoprothrombinemia and bleeding have been reported. Nephritis, glomerulonephritis, interstitial pneumonitis, exfoliative dermatitis, and erythema nodosum have been reported. Reports of a vasculitis syndrome associated with the presence of anti-neutrophilic cytoplasmic antibodies (ANCA) have also been received. Manifestations of ANCA-positive vasculitis may include rapidly progressive glomerulonephritis (crescentic and pauci-immune necrotizing glomerulonephritis), sometimes leading to acute renal failure; pulmonary infiltrates or alveolar hemorrhage; skin ulcers; and leukocytoclastic vasculitis. Minor adverse reactions include skin rash, urticaria, nausea, vomiting, epigastric distress, arthralgia, paresthesias, loss of taste, taste perversion, abnormal loss of hair, myalgia, headache, pruritus, drowsiness, neuritis, edema, vertigo, skin pigmentation, jaundice, sialadenopathy, and lymphadenopathy.
It should be noted that about 10% of patients with untreated hyperthyroidism have leukopenia (white blood cell count of less than 4,000/mm3 ), often with relative granulopenia.
Signs And Symptoms:
Nausea, vomiting, epigastric distress, headache, fever, arthralgia, pruritus, edema, and pancytopenia. Agranulocytosis is the most serious effect. Rarely, exfoliative dermatitis, hepatitis, neuropathies, or CNS stimulation or depression may occur.
No information is available on the following: LD50; concentration of propylthiouracil in biologic fluids associated with toxicity and/or death; the amount of drug in a single dose usually associated with symptoms of overdosage; or the amount of propylthiouracil in a single dose likely to be life-threatening.
Treatment: To obtain up-to-date information about the treatment of overdose, a good resource is the certified Regional Poison Control Center. In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in the patient.
In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s medical status.
Propylthiouracil is administered orally. The total daily dosage is usually given in 3 equal doses at approximately 8-hour intervals.
Adults: The initial dose is 300 mg daily. In patients with severe hyperthyroidism, very large goiters, or both, the initial dose may be increased to 400 mg daily; an occasional patient will require 600 to 900 mg daily initially. The usual maintenance dosage is 100 to 150 mg daily.
Pediatric Patients: Propylthiouracil is generally not recommended for use in the pediatric patient population except in rare instances in which other alternative therapies are not appropriate options. Studies evaluating appropriate dosing regimen have not been conducted in the pediatric population although general practice would suggest initiation of therapy in patients 6 years or older at a dosage of 50 mg daily with careful upward titration based on clinical response and evaluation of TSH and free T4 levels. Although cases of severe liver injury have been reported with doses as low as 50 mg/day, most cases were associated with doses of
300 mg/day and higher.
50 mg — Each white, round, tablet imprinted with  on one side and 348 and partial bisect on the other side contains 50 mg of Propylthiouracil, USP. Tablets are supplied in bottles of 100 (NDC 0228-2348-10).
on one side and 348 and partial bisect on the other side contains 50 mg of Propylthiouracil, USP. Tablets are supplied in bottles of 100 (NDC 0228-2348-10).
1. International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. 1974; 7:67-76.
Dispense in a well-closed container as defined in the USP.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Manufactured by:
Actavis Elizabeth LLC
200 Elmora Avenue
Elizabeth, NJ 07207 USA
40-9158
Revised — January 2011
PROPYLTHIOURACIL TABLETS, USP
(Pro-pil-thi-o-ur-a-sil)
Rx Only
Read this Medication Guide before you start taking propylthiouracil and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment.
What is the most important information I should know about propylthiouracil?
Propylthiouracil can cause serious side effects, including:
Severe liver problems. In some cases, these liver problems can lead to liver failure, the need for liver transplant, or death.
Stop taking propylthiouracil and call your doctor right away if you have:
- fever
- loss of appetite
- nausea
- vomiting
- tiredness
- itchiness
- pain or tenderness in your right upper stomach area (abdomen)
- dark (tea colored) urine
- pale or light colored bowel movements (stools)
- yellowing of your skin or whites of your eyes
What is propylthiouracil?
Propylthiouracil is a prescription medicine used to treat people who have Graves’ disease with hyperthyroidism or toxic multinodular goiter. Propylthiouracil is used when:
- certain other antithyroid medicines do not work well.
- thyroid surgery or radioactive iodine therapy is not a treatment option.
- to decrease symptoms of hyperthyroidism in preparation for a thyroidectomy (removal of the thyroid gland) or radioactive iodine therapy.
Propylthiouracil is not recommended for use in children.
Propylthiouracil may be used when an antithyroid drug is needed during or just before the first trimester of pregnancy.
Who should not take propylthiouracil?
Do not take propylthiouracil if you are allergic to propylthiouracil or any of its ingredients. See the end of this Medication Guide for a complete list of ingredients in propylthiouracil.
What should I tell my doctor before taking propylthiouracil?
Before you take propylthiouracil, tell your doctor if you:
- plan to have surgery.
- have any other medical conditions
- are pregnant or plan to become pregnant. Talk to your doctor right away if you are pregnant or plan to become pregnant.
- Propylthiouracil may cause liver problems, liver failure and death in pregnant women.
- Propylthiouracil may harm your unborn baby.
- are breast-feeding or plan to breast-feed. Propylthiouracil can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you take propylthiouracil.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Propylthiouracil may affect the way other medicines work.
Especially, tell your doctor if you take:
- a blood thinner medicine warfarin sodium (Coumadin®, Jantoven®)
- medicine for heart problems
- medicine for high blood pressure
- Digoxin (Lanoxicaps®, Lanoxin®)
- Theophylline (Elixophyllin®, Theolair®, Theochron®, Theo-24®, Uniphyl®)
Ask your doctor if you are not sure if your medicine is one of these.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take propylthiouracil?
- Take propylthiouracil exactly as your doctor tells you to take it.
- Your doctor may change your dose if needed.
- Propylthiouracil is usually taken 3 times a day (every 8 hours).
- If you take too much propylthiouracil, call your Regional Poison Control Center or go to the nearest hospital emergency room right away.
- If you take too much propylthiouracil you may have the following symptoms:
| • nausea | • fever |
| • vomiting | • joint pain |
| • upper stomach pain or tenderness | • swelling of your body, arms, and legs |
| • headache |
- If you miss a dose of propylthiouracil, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Just take the next dose at your regular time. Do not double your dose.
What are the possible side effects of propylthiouracil?
Propylthiouracil may cause serious side effects, including:
- liver problems. See “What is the most important information I should know about propylthiouracil?”
- low white blood cell counts. This usually happens within the first 3 months of treatment and can be life-threatening. You may have a higher chance of getting an infection when your white blood cell count is low.
Tell your doctor right away if you have:
- a fever
- chills
- sore throat
-
hypothyroidism. Your doctor should do blood tests regularly during treatment to check your thyroid.
- increased bleeding especially with surgical procedures and particularly if you are taking blood thinners.
The most common side effects of propylthiouracil include:
| • skin rash or hives | • headache |
| • nausea | • sleepiness |
| • vomiting | • nerve pain |
| • upper stomach pain or tenderness | • swelling (edema) |
| • joint pain | • dizziness |
| • itching or tingling | • enlarged salivary glands or enlarged lymph nodes |
| • loss or change in taste | |
| • loss of hair | |
| • muscle pain |
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of propylthiouracil. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at
1-800-FDA-1088.
How should I store propylthiouracil?
Store propylthiouracil at 59ºF to 86ºF (15º to 30ºC).
Keep propylthiouracil and all medicines out of the reach of children.
General information about the safe and effective use of propylthiouracil:
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
Do not use propylthiouracil for a condition for which it was not prescribed.
Do not give propylthiouracil to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about propylthiouracil. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about propylthiouracil that is written for health professionals.
For more information call Actavis at 1-800-432-8534.
What are the ingredients in propylthiouracil?
Active ingredient: propylthouracil, USP
Inactive ingredients: lactose monohydrate, corn starch, colloidal silicon dioxide, povidone, pregelatinized starch, and magnesium stearate.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Brands listed are the trademarks of their respective owners.
Manufactured by:
Actavis Elizabeth LLC
200 Elmora Avenue
Elizabeth, NJ 07207 USA
Revised — January 2011
40-9158
(41-1168)
Propylthiouracil Tablets, USP 50 mg are available from Cardinal Health in unit dose packages of 100.
50 mg, unit dose package of 100 tablets, NDC 55154-5996-4
IU43504920611
Cardinal Health
Zanesville, OH 43701
PRINCIPAL DISPLAY PANEL
Propylthiouracil Tablet, USP
50 mg

PRINCIPAL DISPLAY PANEL
NDC: 55154-5996-0
Propylthiouracil Tablets, USP
50 mg
10 Tablets

PRINCIPAL DISPLAY PANEL
NDC: 55154-5996-4
Propylthiouracil Tablets, USP
50 mg
100 Tablets

PropylthiouracilPropylthiouracil TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||