ProstaMate
ProstaMate™ (dinoprost tromethamine)
FULL PRESCRIBING INFORMATION: CONTENTS*
- PROSTAMATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PROSTAMATE INDICATIONS AND USAGE
- WARNINGS
- PRECAUTIONS
- PROSTAMATE ADVERSE REACTIONS
- PROSTAMATE DOSAGE AND ADMINISTRATION
- SAFETY AND TOXICITY
- HOW SUPPLIED
- STORAGE CONDITIONS
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- 30mL Vial Label
- PACKAGE/LABEL INSERT 30mL
- PACKAGE/LABEL SHIPPER LABEL 30mL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-90mL Vial Label
- PACKAGE/LABEL INSERT 90mL LABEL
- PACKAGE/SHIPPER LABEL 90mL
FULL PRESCRIBING INFORMATION
Sterile Solution
Equivalent to 5 mg per mL dinoprost
For intramuscular use in cattle, swine and mares. Net Contents 30mL
For intramuscular use in cattle only. Net Contents 90mL
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
For Use In Animals Only
ANADA 200-253, Approved by FDA
For intramuscular use for estrus synchronization, treatment of unobserved (silent) estrus and pyometra (chronic endometritis) in cattle; for abortion of feedlot and other non-lactating cattle; for parturition induction in swine; and for controlling the timing of estrus in estrous cycling mares and clinically anestrous mares that have a corpus luteum
PROSTAMATE DESCRIPTION
This product contains the naturally occurring prostaglandin F2 alpha (dinoprost) as the tromethamine salt. Each mL contains dinoprost tromethamine equivalent to 5 mg dinoprost: also, benzyl alcohol, 9.45 mg added as preservative. When necessary, pH was adjusted with sodium hydroxide and/or hydrochloric acid. Dinoprost tromethamine is a white or slightly off-white crystalline powder that is readily soluble in water at room temperature in concentrations to at least 200 mg/mL.
CLINICAL PHARMACOLOGY
General Biologic Activity:
Prostaglandins occur in nearly all mammalian tissues. Prostaglandins, especially PGE’s and PGF’s, have been shown, in certain species, to 1) increase at time of parturition in amniotic fluid, maternal placenta, myometrium, and blood, 2) stimulate myometrial activity, and 3) to induce either abortion or parturition. Prostaglandins, especially PGF2α, have been shown to 1) increase in the uterus and blood to levels similar to levels achieved by exogenous administration which elicited luteolysis, 2) be capable of crossing from the uterine vein to the ovarian artery (sheep), 3) be related to IUD induced luteal regression (sheep), and 4) be capable of regressing the corpus luteum of most mammalian species studied to date. Prostaglandins have been reported to result in release of pituitary tropic hormones. Data suggest prostaglandins, especially PGE’s and PGF’s, may be involved in the process of ovulation and gamete transport. Also PGF2α has been reported to cause increase in blood pressure, bronchoconstriction, and smooth muscle stimulation in certain species.
METABOLISM
A number of metabolism studies have been done in laboratory animals. The metabolism of tritium labeled dinoprost (3H PGF2 alpha) in the rat and in the monkey was similar. Although quantitative differences were observed, qualitatively similar metabolites were produced. A study demonstrated that equimolar doses of 3H PGF2 alpha Tham and 3H PGF2 alpha free acid administered intravenously to rats demonstrated no significant differences in blood concentration of dinoprost. An interesting observation in the above study was that the radioactive dose of 3H PGF2 alpha rapidly distributed in tissues and dissipated in tissues with almost the same curve as it did in the serum. The half-life of dinoprost in bovine blood has been reported to be on the order of minutes. A complete study on the distribution of decline of 3 H PGF2 alpha Tham in the tissue of rats was well correlated with the work done in the cow. Cattle serum collected during 24 hours after doses of 0 to 250 mg dinoprost have been assayed by RIA for dinoprost and the 15-keto metabolites. These data support previous reports that dinoprost has a half-life of minutes.
Dinoprost is a natural prostaglandin. All systems associated with dinoprost metabolism exist in the body; therefore, no new metabolic, transport, excretory, binding or other systems need be established by the body to metabolize injected dinoprost.
PROSTAMATE INDICATIONS AND USAGE
Cattle:
ProstaMate™ Sterile Solution is indicated as a luteolytic agent.
ProstaMate™ is effective only in those cattle having a corpus luteum, i.e., those which ovulated at least five days prior to treatment. Future reproductive performance of animals that are not cycling will be unaffected by injection of ProstaMate™.
1. For Intramuscular Use for Estrus Synchronization in Beef Cattle and Non-Lactating Dairy Heifers. ProstaMate™ is used to control the timing of estrus and ovulation in estrous cycling cattle that have a corpus luteum.
Inject a dose of 5 mL ProstaMate™ (25 mg PGF2α) intramuscularly either once or twice at a 10 to 12 day interval. With the single injection, cattle should be bred at the usual time relative to estrus.
With the two injections cattle can be bred after the second injection either at the usual time relative to detected estrus or at about 80 hours after the second injection of ProstaMate™.
Estrus is expected to occur 1 to 5 days after injection if a corpus luteum was present. Cattle that do not become pregnant to breeding at estrus on days 1 to 5 after injection will be expected to return to estrus in about 18 to 24 days.
2. For Intramuscular Use for Unobserved (Silent) Estrus in Lactating Dairy Cows with a Corpus Luteum. Inject a dose of 5 mL ProstaMate™ (25 mg PGF2α) intramuscularly. Breed cows as they are detected in estrus. If estrus has not been observed by 80 hours after injection, breed at 80 hours. If the cow returns to estrus breed at the usual time relative to estrus.
Management Considerations: Many factors contribute to success and failure of reproduction management, and these factors are important also when time of breeding is to be regulated with ProstaMate™ Sterile Solution. Some of these factors are:
a. Cattle must be ready to breed they must have a corpus luteum and be healthy;
b. Nutritional status must be adequate as this has a direct effect on conception and the initiation of estrus in heifers or return of estrous cycles in cows following calving;
c. Physical facilities must be adequate to allow cattle handling without being detrimental to the animal;
d. Estrus must be detected accurately if timed Al is not employed;
e. Semen of high fertility must be used;
f. Semen must be inseminated properly.
A successful breeding program can employ ProstaMate™ effectively, but a poorly managed breeding program will continue to be poor when ProstaMate™ is employed unless other management deficiencies are remedied first.
Cattle expressing estrus following ProstaMate™ are receptive to breeding by a bull. Using bulls to breed large numbers of cattle in heat following ProstaMate™ will require proper management of bulls and cattle.
3. For Intramuscular Use for Treatment of Pyometra (chronic endometritis) in Cattle. Inject a dose of 5 mL ProstaMate™ (25 mg PGF2α) intramuscularly. In studies conducted with dinoprost tromethamine, pyometra was defined as presence of a corpus luteum in the ovary and uterine horns containing fluid but not a conceptus based on palpation per rectum. Return to normal was defined as evacuation of fluid and return of the uterine horn size to 40mm or less based on palpation per rectum at 14 and 28 days. Most cattle that recovered in response to dinoprost tromethamine recovered within 14 days after injection. After 14 days, recovery rate of treated cattle was no different than that of non-treated cattle.
4. For Intramuscular Use for Abortion of Feedlot and Other Non-Lactating Cattle. ProstaMate™ is indicated for its abortifacient effect in feedlot and other non-lactating cattle during the first 100 days of gestation. Inject a dose of 25 mg intramuscularly. Cattle that abort will abort within 35 days of injection.
Commercial cattle were palpated per rectum for pregnancy in six feedlots. The percent of pregnant cattle in each feedlot less than 100 days of gestation ranged between 26 and 84; 80% or more of the pregnant cattle were less than 150 days of gestation. The abortion rates following injection of dinoprost tromethamine increased with increasing doses up to about 25 mg. As examples, the abortion rates, over 7 feedlots on the dose titration study, were 22%, 50%, 71%, 90% and 78% for cattle up to 100 days of gestation when injected IM with dinoprost tromethamine doses of 0,1 (5 mg), 2 (10 mg), 4 (20 mg) and 8 (40 mg) mL, respectively. The statistical predicted relative abortion rate based on the dose titration data, was about 93% for the 5 mL (25 mg) dinoprost tromethamine dose for cattle injected up to 100 days of gestation.
Swine: For intramuscular use for parturition induction in swine. ProstaMate™ Sterile Solution is indicated for parturition induction in swine when injected within 3 days of normal predicted farrowing.
The response to treatment varies by individual animals with a mean interval from administration of 2 mL ProstaMate™ (10 mg dinoprost) to parturition of approximately 30 hours. This can be employed to control the time of farrowing in sows and gilts in late gestation.
Management Considerations: Several factors must be considered for the successful use of ProstaMate™ Sterile Solution for parturition induction in swine. The product must be administered at a relatively specific time (treatment earlier than 3 days prior to normal predicted farrowing may result in increased piglet mortality). It is important that adequate records be maintained on (1) the average length of gestation period for the animals on a specific location, and (2) the breeding and projected farrowing dates for each animal. This information is essential to determine the appropriate time for administration of ProstaMate™.
Mares:
ProstaMate™ Sterile Solution is indicated for its luteolytic effect in mares. This luteolytic effect can be utilized to control the timing of estrus in estrous cycling and clinically anestrous mares that have a corpus luteum in the following circumstances:
1. Controlling Time of Estrus of Estrous Cycling Mares: Mares treated with ProstaMate™ during diestrus (4 or more days after ovulation) will return to estrus within 2 to 4 days in most cases and ovulate 8 to 12 days after treatment. This procedure may be utilized as an aid to scheduling the use of stallions.
2. Difficult-to-Breed Mares: In extended diestrus there is failure to exhibit regular estrous cycles which is different from true anestrus. Many mares described as anestrus during the breeding season have serum progesterone levels consistent with the presence of a functional corpus luteum.
A proportion of "barren", maiden, and lactating mares do not exhibit regular estrous cycles and may be in extended diestrus. Following abortion, early fetal death and resorption, or as a result of "pseudopregnancy", there may be serum progesterone levels consistent with a functional corpus luteum.
Treatment of such mares with ProstaMate™ usually results in regression of the corpus luteum followed by estrus and/or ovulation. In one study with 122 Standardbred and Thoroughbred mares in clinical anestrus for an average of 58 days and treated during the breeding season, behavioral estrus was detected in 81 percent at an average time of 3.7 days after injection with 5 mg dinoprost tromethamine; ovulation occurred an average of 7.0 days after treatment. Of those mares bred, 59% were pregnant following an average of 1.4 services during that estrus.
Treatment of "anestrous" mares which abort subsequent to 36 days of pregnancy may not result in return to estrus due to presence of functional endometrial cups.
WARNINGS
User Safety: Not for human use. Women of childbearing age, asthmatics, and persons with bronchial and other respiratory problems should exercise extreme caution when handling this product. In the early stages, women may be unaware of their pregnancies. Dinoprost tromethamine is readily absorbed through the skin and can cause abortion and bronchiospasms. Accidental spillage on the skin should be washed off immediately with soap and water.
Residue Warnings: No milk discard or pre-slaughter drug withdrawal period is required for labeled uses in cattle. No preslaughter drug withdrawal period is required for labeled uses in swine. Use of this product in excess of the approved dose may result in drug residues.
Warning: Do not use in horses intended for human consumption.
Animal Safety Warnings: Severe localized clostridial infections associated with injection of dinoprost tromethamine have been reported. In rare instances, such infections have resulted in death. Aggressive antibiotic therapy should be employed at the first sign of infection at the injection site whether localized or diffuse.
PRECAUTIONS
- Do not administer intravenously (I.V.) as this route may potentiate adverse reactions.
- No vial stopper should be entered more than 20 times. For this reason, the 90 mL bottle should only be used for cattle. The 30 mL bottle may be used for cattle, swine, or mares.
- As with all parenteral products careful aseptic techniques should be used to decrease the possibility of post-injection bacterial infections. The vial stopper should be cleaned and disinfected prior to needle entry. Only sterile needles should be used and the same needle should not be used more than once.
- Nonsteroidal anti-inflammatory drugs may inhibit prostaglandin synthesis; therefore this class of drugs should not be administered concurrently.
Cattle: Do not administer to pregnant cattle, unless abortion is desired. Cattle administered a progestin would be expected to have a reduced response to ProstaMate™ Sterile Solution.
Swine: Do not administer to sows and/or gilts prior to 3 days of normal predicted farrowing as an increased number of stillbirths and postnatal mortality may result.
Mares: ProstaMate™ Sterile Solution is ineffective when administered prior to day-5 after ovulation. Pregnancy status should be determined prior to treatment since dinoprost tromethamine has been reported to induce abortion and parturition when sufficient doses were administered. Mares should not be treated if they suffer from either acute or subacute disorders of the vascular system, gastrointestinal tract, respiratory system, or reproductive tract.
PROSTAMATE ADVERSE REACTIONS
Cattle: Limited salivation has been reported in some instances.
Swine: The most frequently observed side effects were erythema and pruritus, slight incoordination, nesting behavior, itching, urination, defecation, abdominal muscle spasms, tail movements, hyperpnea or dyspnea, increased vocalization, salivation, and at the 100 mg (10X) dose only, possible vomiting. These side effects are transitory, lasting from 10 minutes to 3 hours, and were not detrimental to the health of the animal.
Mares: The most frequently observed side effects are sweating and decreased rectal temperature. However, these have been transient in all cases observed and have not been detrimental to the animal. Other reactions seen have been increase in heart rate, increase in respiration rate, some abdominal discomfort, locomotor incoordination, and lying down. These effects are usually seen within 15 minutes of injection and disappear within one hour. Mares usually continue to eat during the period of expression of side effects. One anaphylactic reaction of several hundred mares treated with dinoprost tromethamine was reported but was not confirmed.
To report adverse reactions call Bayer HealthCare LLC at 1-800-255-6826.
PROSTAMATE DOSAGE AND ADMINISTRATION
As with any multi-dose vial, practice aseptic techniques in withdrawing each dose. Adequately clean and disinfect the vial stopper prior to entry with a sterile needle and syringe. No vial closure should be entered more than 20 times.
Cattle: ProstaMate™ Sterile Solution is supplied at a concentration of 5 mg dinoprost per mL. ProstaMate™ is luteolytic in cattle at 25 mg (5 mL) administered intramuscularly.
Swine: ProstaMate™ Sterile Solution will induce parturition in swine at 10 mg (2 mL) when injected intramuscularly
Mares:
- Evaluate the reproductive status of the mare.
- Administer a single intramuscular injection of 1 mg per 100 lbs (45.5 kg) body weight which is usually 1 mL to 2 mL ProstaMate™ Sterile Solution.
- Observe for signs of estrus by means of daily teasing with a stallion, and evaluate follicular changes on the ovary by palpation of the ovary per rectum.
- Some clinically anestrous mares will not express estrus but will develop a follicle which will ovulate. These mares may become pregnant if inseminated at the appropriate time relative to rupture of the follicle.
- Breed mares in estrus in a manner consistent with normal manage
SAFETY AND TOXICITY
Laboratory Animals:
Dinoprost was non-teratogenic in rats when administered orally at 1.25, 3.2, 10.0 and 20.0 mg/kg/day from day 6th-15th of gestation or when administered subcutaneously at 0.5 and 1.0 mg/kg/day on gestation days 6, 7 and 8 or 9, 10 and 11 or 12, 13 and 14. Dinoprost was non-teratogenic in the rabbit when administered either subcutaneously at doses of 0.5 and 1.0 mg/kg/day on gestation days 6, 7 and 8 or 9, 10 and 11 or 12, 13 and 14 or 15, 16 and 17 or orally at doses of 0.01, 0.1 and 1.0 mg/kg/day on days 6-18 or 5.0 mg/kg/day on days 8-18 of gestation. A slight and marked embryo lethal effect was observed in dams given 1.0 and 5.0 mg/kg/day respectively. This was due to the expected luteolytic properties of the drug.
A 14-day continuous intravenous infusion study in rats at 20 mg PGF2α per kg body weight indicated prostaglandins of the F series could induce bone deposition. However, such bone changes were not observed in monkeys similarly administered dinoprost tromethamine at 15 mg PGF2α per kg body weight for 14 days.
Cattle: In cattle, evaluation was made of clinical observations, clinical chemistry, hematology, urinalysis, organ weights, and gross plus microscopic measurements following treatment with various doses up to 250 mg dinoprost administered twice intramuscularly at a 10 day interval or doses of 25 mg administered daily for 10 days. There was no unequivocal effect of dinoprost on the hematology or clinical chemistry parameters measured. Clinically, a slight transitory increase in heart rate was detected. Rectal temperature was elevated about 1.5°F through the 6th hour after injection with 250 mg dinoprost, but had returned to baseline at 24 hours after injection. No dinoprost associated gross lesions were detected. There was no evidence of toxicological effects. Thus, dinoprost had a safety factor of at least 10X on injection (25 mg luteolytic dose vs. 250 mg safe dose), based on studies conducted with cattle. At luteolytic doses, dinoprost had no effect on progeny. If given to a pregnant cow, it may cause abortion; the dose required for abortion varies considerably with the stage of gestation.
Induction of abortion in feedlot cattle at stages of gestation up to 100 days of gestation did not result in dystocia, retained placenta or death of heifers in the field studies. The smallness of the fetus at this early stage of gestation should not lead to complications at abortion. However, induction of parturition or abortion with any exogenous compound may precipitate dystocia, fetal death, retained placenta and/or metritis, especially at latter stages of gestation
Swine: In pigs, evaluation was made of clinical observations, food consumption, clinical pathologic determinations, body weight changes, urinalysis, organ weights, and gross and microscopic observations following treatment with single doses of 10, 30, 50 and 100 mg dinoprost administered intramuscularly. The results indicated no treatment related effects from dinoprost treatment that were deleterious to the health of the animals or to their offspring.
Mares: Dinoprost tromethamine was administered to adult mares (weighing 320 to 485 kg; 2 to 20 years old), at the rates of 0, 100, 200, 400, and 800 mg per mare per day for 8 days. Route of administration for each dose group was both intramuscularly (2 mares) and subcutaneously (2 mares). Changes were detected in all treated groups for clinical (reduced sensitivity to pain; locomotor incoordination; hypergastromotility; sweating; hyperthermia; labored respiration), blood chemistry (elevated cholesterol, total bilirubin, LDH, and glucose), and hematology (decreased eosinophils; increased hemoglobin, hematocrit, and erythrocytes) measurements. The effects in the 100 mg dose, and to a lesser extent, the 200 mg dose groups were transient in nature, lasting for a few minutes to several hours. Mares did not appear to sustain adverse effects following termination of the side effects.
Mares treated with either 400 mg or 800 mg exhibited more profound symptoms. The excessive hyperstimulation of the gastrointestinal tract caused a protracted diarrhea, slight electrolyte imbalance (decreased sodium and potassium), dehydration, gastrointestinal irritation, and slight liver malfunction (elevated SGOT, SGPT at 800 mg only). Heart rate was increased but pH of the urine was decreased. Other measurements evaluated in the study remained within normal limits. No mortality occurred in any of the groups. No apparent differences were observed between the intramuscular and subcutaneous routes of administration. Luteolytic doses of dinoprost tromethamine are on the order of 5 to 10 mg administered on one day, therefore, dinoprost tromethamine was demonstrated to have a wide margin of safety. Thus, the 100 mg dose gave a safety margin of 10 to 20X for a single injection or 80 to 160X for the 8 daily injections.
Additional studies investigated the effects in the mare of single intramuscular doses of 0, 0.25, 1.0, 2.5, 3.0, 5.0, and 10.0 mg dinoprost tromethamine. Heart rate, respiration rate, rectal temperature, and sweating were measured at 0, 0.25, 0.50, 0.75, 1.0, 1.5, 2.0, 3.0, 4.0, 5.0, and 6.0 hr. after injection. Neither heart rate nor respiration rates were significantly altered (P > 0.05) when compared to contemporary control values. Sweating was observed for 0 of 9, 2 of 9, 7 of 9, 9 of 9, and 8 of 9 mares injected with 0.25, 1.0, 2.5, 3.0, 5.0, or 10.0 mg dinoprost tromethamine, respectively. Sweating was temporary in all cases and was mild for doses of 3.0 mg or less but was extensive (beads of sweat over the entire body and dripping) for the 10 mg dose. Sweating after the 5.0 mg dose was intermediate between that seen for mares treated with 3.0 and 10.0 mg. Sweating began within 15 minutes after injection and ceased by 45 to 60 minutes after injection. Rectal temperature was decreased during the interval 0.5 until 1.0, 3 to 4, or 5 hours after injection for 0.25 and 1.0 mg, 2.5 and 3.0, or 5.0 and 10.0 mg dose groups, respectively. Average rectal temperature during the periods of decreased temperature was on the order of 97.5 to 99.6, with the greatest decreases observed in the 10 mg dose group.
HOW SUPPLIED
ProstaMate™ Sterile Solution is available in 30 and 90 mL vials
STORAGE CONDITIONS
Store at controlled room temperature 20° - 25°C (68° - 77°F). Protect from freezing.
Manufactured for Bayer HealthCare LLC, Shawnee Mission, KS 66201
Bayer (reg’d), the Bayer Cross (reg’d) and ProstaMate are trademarks of Bayer.
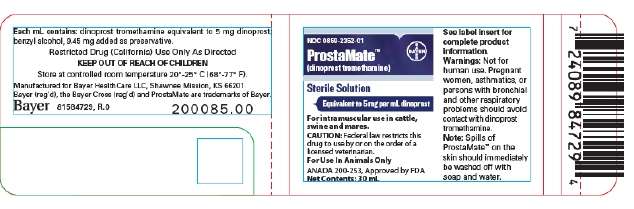
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL- 30mL Vial Label
ProstaMate™
dinoprost tromethamine
Sterile Solution
Equivalent to 5 mg per mL dinoprost
For intramuscular use in cattle, swine and mares.
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
For Use in Animals Only
ANADA 200-253, Approved by FDA
Net Contents 30mL
81584729, R.0 200085.00
PACKAGE/LABEL INSERT 30mL

PACKAGE/LABEL SHIPPER LABEL 30mL

PACKAGE/LABEL PRINCIPAL DISPLAY PANEL-90mL Vial Label
ProstaMate™
dinoprost tromethamine
Sterile Solution
Equivalent to 5 mg per mL dinoprost
For intramuscular use in cattle only
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
For Use in Animals Only
ANADA 200-253, Approved by FDA
Net Contents 90mL
81584710, R.0 200086.00

PACKAGE/LABEL INSERT 90mL LABEL

PACKAGE/SHIPPER LABEL 90mL

ProstaMatedinoprost tromethamine INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||