Provocholine
Methapharm Inc.
Methapharm Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Provocholine (methacholine chloride)
PROVOCHOLlNE® (METHACHOLINE CHLORIDE POWDER FOR INHALATION) IS A BRONCHOCONSTRICTOR AGENT FOR DIAGNOSTIC PURPOSES ONLY AND SHOULD NOT BE USED AS A THERAPEUTIC AGENT. PROVOCHOLlNE® INHALATION CHALLENGE SHOULD BE PERFORMED ONLY UNDER THE SUPERVISION OF A PHYSICIAN TRAINED IN AND THOROUGHLY FAMILIAR WITH ALL ASPECTS OF THE TECHNIQUE OF METHACHOLINE CHALLENGE, ALL CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS, AND THE MANAGEMENT OF RESPIRATORY DISTRESS. EMERGENCY EQUIPMENT AND MEDICATION SHOULD BE IMMEDIATELY AVAILABLE TO TREAT ACUTE RESPIRATORY DISTRESS.
PROVOCHOLlNE® SHOULD BE ADMINISTERED ONLY BY INHALATION. SEVERE BRONCHOCONSTRICTION AND REDUCTION IN RESPIRATORY FUNCTION CAN RESULT FROM THE ADMINISTRATION OF PROVOCHOLlNE®. PATIENTS WITH SEVERE HYPERREACTIVITY OF THE AIRWAYS CAN EXPERIENCE BRONCHOCONSTRICTION AT A DOSAGE AS LOW AS 0.025 MG/ML (0.125 CUMULATIVE UNITS). IF SEVERE BRONCHOCONSTRICTION OCCURS, IT SHOULD BE REVERSED IMMEDIATELY BY THE ADMINISTRATION OF A RAPID ACTING INHALED BRONCHODILATOR AGENT (BETAAGONIST). BECAUSE OF THE POTENTIAL FOR SEVERE BRONCHOCONSTRICTION, PROVOCHOLlNE® CHALLENGE SHOULD NOT BE PERFORMED IN ANY PATIENT WITH CLINICALLY APPARENT ASTHMA, WHEEZING, OR VERY LOW BASELINE PULMONARY FUNCTION TESTS (e.g., FEV1 LESS THAN 1 TO 1.5 LITER OR LESS THAN 70% OF THE PREDICTED VALUES). PLEASE CONSULT STANDARD NOMOGRAMS FOR PREDICTED VALUES1
Provocholine® (methacholine chloride powder for inhalation) is a parasympathomimetic (cholinergic)) bronchoconstrictor agent to be administered in solution only, by inhalation, for diagnostic purposes. Each 20 mL vial contains 100 mg of methacholine chloride powder which is to be reconstituted with 0.9% sodium chloride injection or 0.9% sodium chloride injection containing 0.4% phenol (pH 7.0). See DOSAGE AND ADMINISTRATION for dilution procedures, concentrations and schedule of administration.
Chemically, methacholine chloride (the active ingredient) is 1-propanaminium, 2-(acetyloxy)N,N,N, - trimethyl,-chloride. It is a white to practically white deliquescent compound, soluble in water. Methacholine chloride has an empirical formula of C8H18ClN02, a calculated molecular weight of 195.69, and the following structural formula:
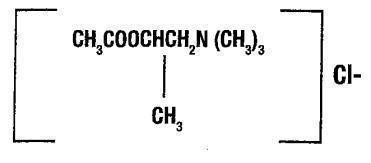
Methacholine chloride is the ß-methyl homolog of acetylcholine and differs from the latter primarily in its greater duration and selectivity of action. Bronchial smooth muscle contains significant parasympathetic (cholinergic) innervation.
Bronchoconstriction occurs when the vagus nerve is stimulated and acetylcholine is released from the nerve endings. Muscle constriction is essentially confined to the local site of release because acetylcholine is rapidly inactivated by acetylcholinesterase.
Compared with acetylcholine, methacholine chloride is more slowly hydrolyzed by acetylcholinesterase and is almost totally resistant to inactivation by nonspecific cholinesterase or pseudocholinesterase. When a sodium chloride solution containing methacholine chloride is inhaled, subjects with asthma are markedly more sensitive to methacholine-induced bronchoconstriction than are healthy subjects. This difference in response is the pharmacologic basis for the Provocholine® (methacholine chloride powder for inhalation) inhalation diagnostic challenge. However, it should be recognized that methacholine challenge may occasionally be positive after influenza, upper respiratory infections or immunizations, in very young or very old patients, or in patients with chronic lung disease (cystic fibrosis, sarcoidosis, tuberculosis, chronic obstructive pulmonary disease). The challenge may also be positive in patients with allergic rhinitis without asthma, in smokers, in patients after exposure to air pollutants, or in patients who have had or will in the future develop asthma.
There are no metabolic and pharmacokinetic data available on methacholine chloride.
Provocholine®(methacholine chloride powder for inhalation) is indicated for the diagnosis of bronchial airway hyperreactivity in subjects who do not have clinically apparent asthma.
Provocholine® (methacholine chloride powder for inhalation) is contraindicated in patients with known hypersensitivity to this drug or to other parasympathomimetic agents.
Repeated administration of Provocholine® by inhalation other than on the day that a patient undergoes challenge with increasing doses is contraindicated.
Inhalation challenge should not be performed in patients receiving any beta-adrenergic blocking agent because in such patients responses to methacholine chloride can be exaggerated or prolonged, and may not respond as readily to accepted modalities of treatment (see WARNINGS box).
General:
Administration of Provocholine® (methacholine chloride powder for inhalation) to patients with epilepsy, cardiovascular disease accompanied by bradycardia, vagotonia, peptic ulcer disease, thyroid disease, urinary tract obstruction or other condition that could be adversely affected by a cholinergic agent should be undertaken only if the physician feels benefit to the individual outweighs the potential risks.
Information for Patients:
To assure the safe and effective use of Provocholine® inhalation challenge, the following instructions and information should be given to patients:
- Patients should be instructed regarding symptoms that may occur as a result of the test and how such symptoms can be managed.
- female patient should inform her physician if she is pregnant, or the date of her last onset of menses, or the date and result of her last pregnancy test. (See PRECAUTIONS: Pregnancy.)
Carcinogenesis, Mutagenesis, Impairment of Fertility:
There have been no studies with methacholine chloride that would permit an evaluation of its carcinogenic or mutagenic potential or of its effect on fertility.
Pregnancy:
Teratogenic Effects: Pregnancy Category C. Animal reproduction studies have not been conducted with methacholine chloride. It is not known whether methacholine chloride can cause fetal harm when administered to a pregnant patient or affect reproductive capacity. Methacholine chloride should be given to a pregnant woman only if clearly needed.
IN FEMALES OF CHILDBEARING POTENTIAL, PROVOCHOLlNE® INHALATION CHALLENGE SHOULD BE PERFORMED EITHER WITHIN TEN DAYS FOLLOWING THE ONSET OF MENSES OR WITHIN 2 WEEKS OF A NEGATIVE PREGNANCY TEST.
Nursing Mothers:
Provocholine® inhala tion challenge should not be administered to a nursing mother since it is not known whether methacholine chloride when inhaled is excreted in breast milk.
Pediatric Use:
The safety and efficacy of Provocholine® inhalation challenge have not been established in children below the age of 5 years.
Adverse reactions associated with 153 inhaled methacholine chloride challenges include one occurrence each of headache, throat irritation, Iightheadedness and itching.
Provocholine® (methacholine chloride powder for inhalation) is to be administered only by inhalation. When administered orally or by injection, methacholine chloride is reported to be associated with nausea and vomiting, substernal pain or pressure, hypotension,fainting and transient complete heart block. (See OVERDOSAGE.)
Provocholine® (methacholine chloride powder for inhalation) is to be administered only by inhalation. When administered orally or by injection, overdosage with methacholine chloride can result in a syncopal reaction, with cardiac arrest and loss of consciousness. Serious toxic reactions should be treated with 0.5 mg to 1 mg of atropine sulfate, administered IM or IV.
The acute (24 hour) oral LD50 of methacholine chloride and related compounds is 1100 mg/kg in the mouse and 750 mg/kg in the rat.
Cynomolgus monkeys were exposed to a 2% (20 mg/mL) aerosol of methacholine chloride in acute (10 minute) and subchronic (7 day) inhalation toxicity studies. In the former study, animals exposed to the aerosol for up to 10 minutes demonstrated an increase in respiratory rate and decrease in tidal volume after 30 seconds. These changes peaked at 2 minutes and were followed by a rise in pulmonary resistance and a decrease in compliance. Pulmonary function returned to normal 20 to 25 minutes after exposure ended. In the 7 day study, monkeys were given daily inhalations equivalent to the maximum and roughly five times the maximum standard human dose. Although the typical pulmonary response/ recovery sequence was observed, distinct changes in airway resistance were noted at the end of the study. These changes were not rapidly reversed in the maximum equivalent standard dose group, which was observed for 9 weeks.
Before Provocholine® (methacholine chloride powder for inhalation) Inhalation challenge is begun, baseline pulmonary function tests must be performed. A subject to be challenged must have an FEV1 of at least 70% of the predicted value.
The target level for a positive challenge is a 20% reduction in the FEV1 compared with the baseline value after inhalation of the control sodium chloride solution (Note: Use the same diluent that the Provocholine® powder has been reconstituted with for the baseline spirometry). This target value should be calculated and recorded before Provocholine® challenge is started.
Dilutions: (Note: Do not inhale powder. Do not handle this material if you have asthma or hay fever.) All dilutions should be made with 0.9% sodium chloride injection or 0.9% sodium chloride injection containing 0.4% phenol (pH 7.0) using sterile, empty USP Type I borosilcate glass vials. After adding the sodium chloride solution, shake each vial to obtain a clear solution (Note: When preparing dilutions, use only the same kind of diluent to prepare all concentrations).
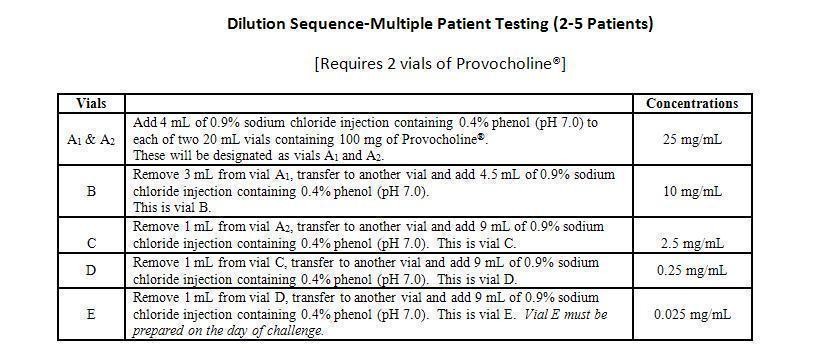
Dilutions A through D should be stored at 36° to 46°F (2° to 8°C) in a refrigerator and can be stored for not more than 2 weeks. [The unreconstituted powder should be stored at 59°F to 86°F (15° to 30°C)]. After this time, discard the vials and prepare new dilutions. Freezing does not affect the stability of dilutions A through D. Vial E must be prepared on the day of challenge.
A Sterile bacterial-retentive filter (porosity 0.22µm) should be used when transferring a solution from each vial (at least 2mL) to a nebulizer.
Procedure: A standardized procedure for inhalation has been developed.
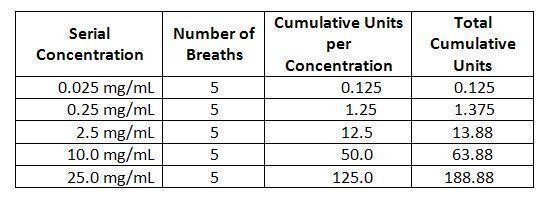
The challenge is performed by giving a subject ascending serial concentrations of Provocholine®. At each concentration, five breaths are administered by a nebulizer that permits intermittent delivery time of 0.6 seconds by a breath-actuated timing device (dosimeter).
At each of five inhalations of a serial concentration, the subject begins at functional residual capacity (FRC) and slowly and completely inhales the dose delivered. Within 5 minutes, FEV1 values are determined. The procedure ends either when there is a 20% or greater reduction in FEV1 compared with the baseline sodium chloride solution value (i.e., a positive response) or if 188.88 total cumulative units has been administered (see table below) and the FEV1 has been reduced by 14% or less (i.e., a negative response). If there is a reduction of 15% to 19% in the FEV1 compared with baseline, either the challenge may be repeated at that concentration or a higher concentration may be given as long as the dosage administered does not result in total cumulative units exceeding 188.88.
The following is a suggested schedule for the administration of Provocholine® (methacholine chloride powder for inhalation) challenge. Cumulative units are calculated by multiplying the number of breaths by the concentration administered.
Total cumulative units are the sum of cumulative units for each concentration administered.
An inhaled beta-agonist may be administered after Provocholine® challenge to expedite the return of the FEV1 to baseline and to relieve the discomfort of the subject. Most patients revert to normal pulmonary function within 5 minutes following bronchodilators or within 30 to 45 minutes without any bronchodilator.
20mL amber vial containing 100mg methacholine chloride powder which is to be reconstituted with 0.9% sodium chloride injection containing 0.4% phenol (pH 7.0) – boxes of 6 (NDC 64281-100-06). Store the powder at 59° to 86°F (15° to 30°C). Refrigerate the reconstituted solutions (dilutions A-D) at 36° to 46°F (2° to 8°C) for not more than 2 weeks. Dilution E must be prepared on the day of the challenge.
- Morris JF, Koski WA, Johnson LC. Spirometric standards for healthy non-smoking adults. Am Rev Resp Dis. Jan 1971; 103: 57-67.

Methapharm Inc.
81 Sinclair Boulevard
Brantford, Ontario, Canada N3S 7X6
Toll free: 800.287.7686
Tel: 519.751.3602
Fax: 519.754.9149
Email: sales@methapharm.com
Web: www.methapharm.com

Provocholine® 100mg- Carton Label
Provocholine® 100mg- Vial Label
Provocholinemethacholine chloride POWDER, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||