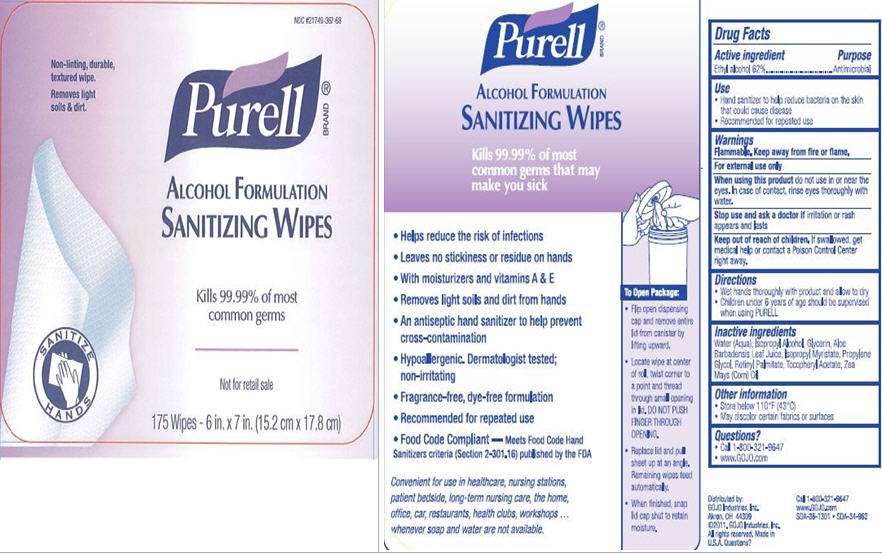
Purell Alcohol Formulation
PURELL Alcohol Formulation Sanitizing Wipes
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Purell Alcohol Formulation Uses
- Warnings
- Directions
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Ethyl Alcohol 62%
Purpose
Antimicrobial
Purell Alcohol Formulation Uses
- Hand sanitizer to help reduce bacteria on the skin that could cause disease
- Recommended for repeated use
Warnings
Flammable. Keep away from fire or flame.
For external use only
do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
if irritation or rash appears and lasts
Keep Out of Reach of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands thoroughly with product and allow to dry
- Children under 6 years of age should be supervised when using PURELL
Inactive ingredients
Water (Aqua), Isopropyl Alcohol, Glycerin, Aloe Barbadensis Leaf Juice, Isopropyl Myristate, Propylene Glycol, Retinyl Palmitate, Tocopheryl Acetate, Zea Mays (Corn) Oil
- Store below 110°F (43°C)
- May discolor certain fabrics or surfaces
Questions?
- Call 1-800-321-0647
- www.GOJO.com
Package/Label Principal Display Panel
NDC #21749-367-68
Non-linting, durable, textured wipe.
Removes light soils & dirt.
Purell
ALCOHOL FORMLATION
SANITIZING WIPES
Kills 99.99% of most common germs
Not for retail sale
175 Wipes - 6 in. x 7 in. (15.2 cm x 17.8 cm)
Canister Label
Purell Alcohol Formulationalcohol SWAB
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!