Home – Pyridostigmine Bromide
Pyridostigmine Bromide
REMEDYREPACK INC.
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
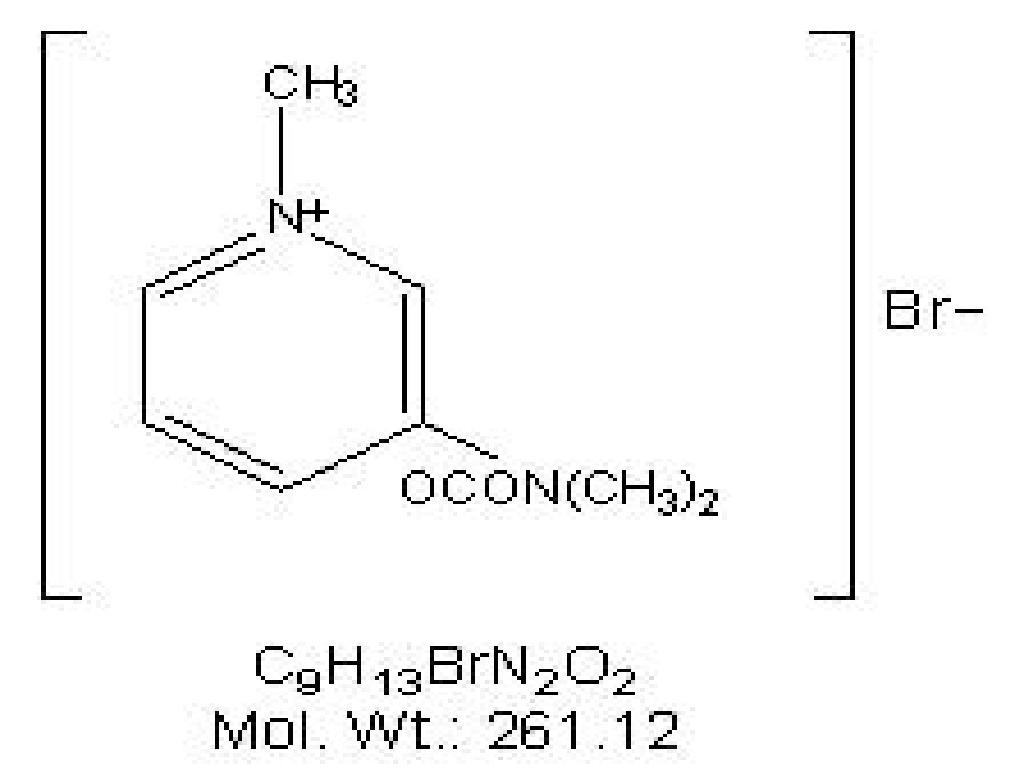
PYRIDOSTIGMINE BROMIDE DESCRIPTION
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
PYRIDOSTIGMINE BROMIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
PREGNANCY
PEDIATRIC USE
PYRIDOSTIGMINE BROMIDE ADVERSE REACTIONS
DOSAGE & ADMINISTRATION
Dosage
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Pyridostigmine Bromide
Pyridostigmine Bromide TABLET
Product Information
|
|
Product Type
|
Human prescription drug label |
Item Code (Source)
|
NDC:49349-560(NDC:0115-3511) |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
PYRIDOSTIGMINE BROMIDE pyridostigmine |
|
60 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
white |
10 mm |
G3511 |
ROUND |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:49349-560-20 |
100 in 1 CANISTER |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
ANDA |
ANDA040502 |
2011-10-04 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!

