PYtest
PYtest (C-Urea Capsules)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- PYtest Indications and Usage
- Contraindications
- Warnings
- Precautions
- Side Effects
- Overdosage
- Dosage and Administration
- Dosage
- Procedural Notes
- Quality Control
- Results
- Expected Values
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - PYtest* Capsule Label
FULL PRESCRIBING INFORMATION
Description
PYtest
PYtest
Data on C-urea
Structural Formula (14C-urea): NH2 14CONH2
Radiation emission: beta-emission, 49 keVmean, 156 keVmax, no other emissions
External emission: No external radiation hazard. Low-energy beta emissions only.
Maximum range of 0.3 mm in water.
Radiological Half-life: 5730 years
Maximum effective dose equivalent (EDE) : 0.3 mrem/µCi
Clinical Pharmacology
The urease enzyme is not present in mammalian cells, so the presence of urease in the stomach is evidence that bacteria are present. The presence of urease is not specific for H. pylori, but other bacteria are not usually found in the stomach. The principle of the breath test is shown in Figure 1.
Figure 1: Principle of Breath Test
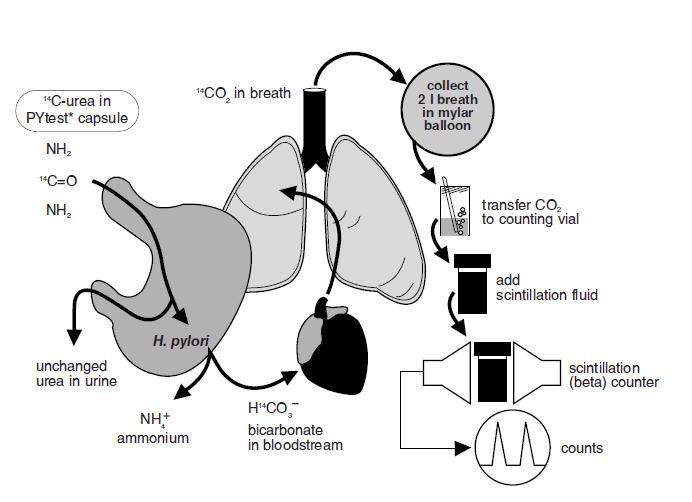
To detect H. pylori, urea labeled with 14C is swallowed by the patient. If gastric urease from H. pylori is present, urea is split to form CO2 and NH3 at the interface between the gastric epithelium and lumen and 14CO2 is absorbed into the blood and exhaled in the breath.
Following ingestion of the capsule by a patient with H. pylori, 14CO2 excretion in the breath peaks between 10 and 15 minutes and declines thereafter with a biological half-life of about 15 minutes. 14C-urea that is not hydrolyzed by H. pylori is excreted in the urine with a half-life of approximately 12 hours. About 10% of the 14C remains in the body at 72 hours and is gradually excreted with a biological half-life of 40 days.
Clinical Studies
Two studies were performed. In both studies, patients with gastrointestinal symptoms underwent the breath test and an endoscopy. During the endoscopy, biopsy samples were taken from the antral gastric mucosa for histological analysis (2 samples, Giemsa stain) and rapid urease test (1 sample, CLOtest
Results were reported as disintegrations per minute (DPM). Analysis for accuracy used the ten minute breath sample. A breath sample DPM <50 was defined as a negative result. DPM ≥ 200 was defined as a positive result. DPM in the range of 50–199 was classified as indeterminate.
STUDY 1
Of 186 patients who had histopathology and CLOtest

Histology and CLOtest |
||||||
|---|---|---|---|---|---|---|
| H. pylori | Positive | Negative | Total | |||
Notes:
PYtest  |
||||||
ppv = positive predictive value (true positive divided by total PYtest |
||||||
npv = negative predictive value (true negative divided by total PYtest |
||||||
PYtest |
Positive | 51 | 8 | 59 | ppv. | 86% |
| (DPM | Indeterminate | 1 | 8 | 9 | ||
| 10 min.) | Negative | 1 | 117 | 118 | npv. | 99% |
| Total | 53 | 133 | 186 | |||
| sensitivity | specificity | |||||
| 96% | 88% | |||||
STUDY 2
Breath tests were performed on 436 outpatients attending gastroenterology practices at sites in the United States. Seventy-six patients (40 men, 36 women) who had histology and CLOtest
Histology and CLOtest |
||||||
|---|---|---|---|---|---|---|
| H. pylori | Positive | Negative | Total | |||
| Notes: PYtest at 10 min. was compared to the gold standard of biopsy results in which histology and CLOtest concurred. Patients who did not have both biopsy tests performed, or in whom the tests differed, were excluded from analysis. There was no statistical difference in test accuracy based on gender of patient. | ||||||
ppv = positive predictive value (true positive divided by total PYtest |
||||||
npv = negative predictive value (true negative divided by total PYtest |
||||||
PYtest |
Positive | 22 | 0 | 22 | ppv. | 100% |
| (DPM | Indeterminate | 4 | 2 | 6 | ||
| 10 min.) | Negative | 1 | 47 | 48 | npv. | 98% |
| Total | 27 | 49 | 76 | |||
| sensitivity | specificity | |||||
| 82% | 96% | |||||
PYtest Indications and Usage
PYtest
Contraindications
None
Warnings
None
Precautions
General
After the patient ingests the 14C-urea capsule, the sample collected for test purposes is for in vitro diagnostic use only.
A false positive test could occur in patients who have achlorhydria. Very rarely, a false positive test may occur due to urease associated with Helicobacters other than H. pylori (i.e. Helicobacter heilmanni).
Limitations of the Test
- The test has been evaluated in outpatients before elective endoscopy.
- Test results should be evaluated with clinical signs and patient history when diagnosing H. pylori infection.
- The performance characteristics of the test have not been established for monitoring the efficacy of antimicrobial therapies for the treatment of H. pylori infection.
- A negative result does not completely rule out the possibility of H. pylori infection. If clinical signs and patient history suggest H. pylori infection, repeat the PYtest

Registered Trademark or Trademark of Kimberly-Clark Worldwide, Inc. or use an alternative diagnostic method.
Radioactivity
Persons concerned about very low doses of radioactivity may postpone the test or may decide to use an alternative means of diagnosis. The test produces radiation exposure equal to 24 hours of normal background. In animal experiments, such low doses of radiation do not carry measurable risk.
Preclinical studies were not conducted on 14C-urea. The estimated dose equivalent received from a single administration of PYtest
Information for Patients
It is necessary for the patient to fast for 6 hours before the test. The patient should also be off antibiotics and bismuth for 1 month, and proton pump inhibitors and sucralfate for 2 weeks prior to the test. Instruct the patient not to handle the capsule directly as this may interfere with the test result. The capsule should be swallowed intact. Do not chew the capsule.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been conducted with 14C-urea to evaluate its potential for carcinogenicity, impairment of fertility, or mutagenicity.
Drug Interactions
Antibiotics, proton pump inhibitors, sucralfate, and bismuth preparations are known to suppress H. pylori. Ingestion of antibiotics or bismuth within 4 weeks and proton pump inhibitors or sucralfate within 2 weeks prior to performing the test may give false negative results.
Pregnancy
Pregnancy category C
Animal reproduction studies have not been conducted with PYtest


Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PYtest
Pediatric Use
Clinical studies in children have not been conducted. However, PYtest
Side Effects
No adverse reactions were reported in clinical trials.
Overdosage
Risk from radiation is negligible even with a 1000 capsule overdose (0.3 rem). If overdose occurs, the patient may drink one glass of water (150 ml) every hour to hasten excretion of the isotope. Maximum excretion of urea is achieved at a urine output of ≥ 2.0 ml/min.
Dosage and Administration
Materials Needed but not Provided
FOR ANALYSIS AT TEST SITE
BREATH SAMPLE COLLECTED INTO BALLOON
(RECOMMENDED METHOD; SEE FIGURE 2)
- Breath test report form
- Stopwatch/Timer capable of timing an interval up to 10 minutes
- Marking pen
- 1 – 20 ml scintillation vial
- Breath transfer pump
- Pipette (10 ml) for measuring fluids
- Collection fluid (2.5 ml/vial)
- Scintillation fluid (10 ml/vial)
- 1 – Mylar collection balloon
- 1 – Straw
- 2 – Needles
- 2 – 30 ml medicine cups
- Water (40 ml)
- Any gas pump that is airtight and has a flow rate between .5 and 1 liter per minute may be used.
Figure 2: Other Components
FOR ANALYSIS ON SITE
BREATH SAMPLE COLLECTED INTO VIAL
CAUTION: Kimberly-Clark does not endorse breath sample collection by this method because patients might come into direct contact with the hyamine.
- Breath test report form
- Stopwatch/Timer capable of timing an interval up to 10 minutes
- Safety trap (Figure 3)
- 1 – Straw
- 1 – 20 ml scintillation vial
- Marking pen
- Pipette (10 ml) for measuring fluids
- Collection fluid (2.5 ml/vial)
- Scintillation fluid (10 ml/vial)
Figure 3: Typical Safety Trap
Dosage
One PYtest
Procedural Notes
- Inform the patient to fast for 6 hours prior to the test.
- The patient should be off antibiotics and bismuth for 1 month, and proton pump inhibitors and sucralfate for 2 weeks prior to the test.
- Have patient sitting at rest while doing the test.
- The capsule should not be handled directly as this may interfere with the test result.
- To avoid contamination by bacteria in the mouth, the capsule should be swallowed intact. Do not chew capsule.
Step by Step Procedure for Balloon
| Before the test |
|
| Minus 1 minute |
|
| 0 minute |
|
| 3 minutes | Ask the patient to drink another 20 ml of lukewarm water (in case the capsule may have lodged in the esophagus and not yet reached the gastric mucosa). |
| 10 minutes |
|
| After sample collection |
See test analysis procedure. |
| CAUTION: Kimberly-Clark does not endorse the collection of breath samples by this method for patient safety reasons. | |
| Before the test |
|
| Minus 2 minutes |
Attach a straw to the safety trap (Figure 3). |
| Minus 1 minute |
|
| 0 minute |
|
| 3 minutes | Ask the patient to drink another 20 ml of lukewarm water (in case the capsule may have lodged in the esophagus and not yet reached the gastric mucosa). |
| 10 minutes | Ask the patient to hold his/her breath for 5–10 seconds, then blow bubbles into the collection fluid via the safety trap. The patient should blow bubbles until the fluid turns clear. Put lid on vial. |
| After sample collection |
See test analysis procedure. |
Test Analysis Procedure
General Information
- The collection fluid contains hyamine, methanol and a pH indicator. Hyamine is a corrosive caustic alkali. If you are using the vial method and collecting breath directly into the collection fluid you must supervise the patient and ensure that a safety trap is used.
- The amount of hyamine (1 ml of 1/m) and methanol (2.5 ml) in one collection vial is not sufficient to cause serious poisoning. Local irritation to skin or mucous membranes is likely when the solution is blue. After collecting CO2 the collection fluid turns clear. At this point it is less dangerous because the pH is near neutral.
- If the collection fluid splashes onto the skin or eyes, wash immediately with water. If the collection fluid is accidentally ingested, wash the mouth with water and have the patient drink 250 ml of water immediately. Consult your poison information center for further facts on hyamine.
- Scintillation fluid comes in many formulations. It is usually a mixture of toluene and various cyclic hydrocarbons. It is flammable. Biodegradable formulations do exist. Consult your supplier if you have questions.
BALLOON READ ON-SITE:
- Add 2.5 ml collection fluid to each vial.
- Label the scintillation vial lid to match the label on the balloon.
- Attach a needle to the inlet and outlet tubes on the pump.
- Turn on pump. Allow to run for about 15 seconds.
- Put needle from the rigid outlet tube in a vial until it makes bubbles in the collection fluid.
- Pierce the label of a filled balloon with the other needle and hold it in a stable position. (Do not pierce the balloon anywhere else or it may tear. Do not squeeze the balloon.)
- After 2–3 minutes the collection fluid should turn colorless.
- After the collection fluid is colorless, remove the needles from the balloon and collection fluid and discard according to your facility protocol.
- Change needles between patients.
- Turn off pump after last patient sample is transferred.
- Double check that the label on the vial lid matches the label on the balloon.
- Add 10 ml scintillation fluid to each vial and mix fluid.
- Count the sample in liquid scintillation counter for 5 minutes or as directed by the liquid scintillation counter manufacturer. (Note that values of 50–300 DPM can occur immediately after the addition of the scintillation fluid due to chemiluminescence. Chemiluminescence decays rapidly over an hour or two and will reveal itself by falling counts. Read the sample repeatedly until values 10 minutes apart are similar. If DPM is still 50–300, you should allow the sample to settle for 12–24 hours before re-testing.)
- Include a standard and a blank control vial in each run.
VIALS READ ON-SITE
See steps 12–14 under "Balloon read on-site."
Quality Control
A minimum of 1 mmol of CO2 is required to perform analysis of a breath sample. The amount of breath required to provide 1 mmol of CO2 varies depending on the amount of CO2 the patient is producing. Since a full balloon typically contains at least 1 mmol of CO2, the balloon should be completely filled.
Results
| Interpretation of results (10 minute sample) | |
| < 50 DPM | Negative for H. pylori |
| 50–199 DPM | Indeterminate for H. pylori |
| ≥ 200 DPM | Positive for H. pylori |
The indeterminate result should be evaluated by repeating the PYtest

The cutoff point of 50 DPM was determined to be the mean +3SD of results obtained in patients who did not have H. pylori.
DPM = Disintegrations per minute
| Factor | Result | Comment |
|---|---|---|
| Recent antibiotic or bismuth (Pepto-Bismol, etc.) |
false neg. |
Relapse of partially treated Hp may take 1–4 weeks. |
| Omeprazole (or other proton pump inhibitors) | false neg. |
These agents suppress Hp in 40% of patients. Discontinuefor at least 2 weeks before performing the PYtest |
| Resective gastric surgery | false neg. |
Isotope may empty rapidly from the stomach. |
| Resective gastric surgery | false pos. |
Patient may be achlorhydric and have bacterial overgrowth (non-Hp urease). |
| Food in stomach (also bezoar, gastroparesis) | unknown | Isotope may not come into contact with gastric mucosa Patient may be achlorhydric and/or have bacterial overgrowth (non-Hp urease). |
Expected Values
As shown in Figure 4 approximately 30% of patients tested will be positive for H. pylori.
Figure 4: Histogram showing DPM distribution for the PYtest
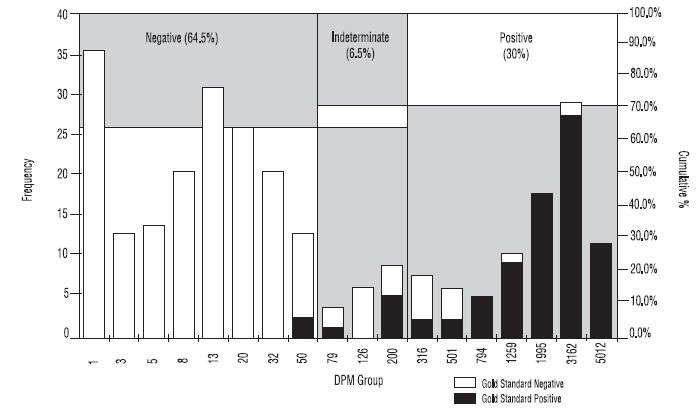
*Note: DPM groupings were calculated on a logarithmic scale. Empty DPM groupings were not included. Chart includes all patients from Studies 1 and 2. Frequency of DPM group includes samples with DPM < Group Name.
-
-

Registered Trademark or Trademark of Kimberly-Clark Worldwide, Inc.
If the capsule is damaged or appears abnormal in any way, it may give inaccurate results.
HOW SUPPLIED
PYtest
PYtest

The PYtest
PYtest
Rx Only (USA)
U.S. Patent Nos. 4,748,113; 4,830,010
| Kimberly-Clark | Distributed in the U.S. by Kimberly-Clark Global Sales, LLC, Roswell, GA 30076 USA In USA, please call 1-800-KCHELPS • www.kchealthcare.com Kimberly-Clark, Roswell, GA 30076 USA Kimberly-Clark N.V., Belgicastraat 13, 1930 Zaventem, Belgium Sponsored in Australia by Kimberly-Clark Australia Pty Limited; 52 Alfred Street Milsons Point, NSW 2061 • 1-800-101-021 |
| ©2003 KCWW. All rights reserved. 14-63-133-0-00/70080916 |
|
PRINCIPAL DISPLAY PANEL - PYtest* Capsule Label
Kimberly-Clark* PYtest*
14C-Urea Breath Test Capsules
for the detection of Helicobacter pylori
Contents – 10 PYtest* Capsules
each containing 1 µCi 14C-Urea
For Dosage Information, Please See Package Insert
14C-Urea (5730yr1/2, 156keV[max] β-emission)
For In Vitro Diagnostic Use
Rx only (USA)
Store at 15°– 30°C (59°– 86°F)
REF 60442
U.S. Patent No. 4830010

PYtestUrea, C-14 CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||