PYtest
PYtest Kit (C-Urea Breath Test)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Clinical Studies
- PYtest Indications and Usage
- Contraindications
- Warnings
- Precautions
- Side Effects
- Overdosage
- Dosage and Administration
- How Supplied
- PRINCIPAL DISPLAY PANEL - PYtest* Kit Label
FULL PRESCRIBING INFORMATION
Description
PYtest
The PYtest

Data on C-urea
Structural Formula (14C-urea): NH2 14CONH2
Radiation emission: beta-emission, 49 keVmean, 156 keVmax, no other emissions
External emission: No external radiation hazard. Low-energy beta emissions only. Maximum range of 0.3 mm in water.
Radiological Half-life: 5730 years
Maximum effective dose equivalent (EDE) : 0.3 mrem/µCi
Clinical Pharmacology
The urease enzyme is not present in mammalian cells, so the presence of urease in the stomach is evidence that bacteria are present. The presence of urease is not specific for H.pylori, but other bacteria are not usually found in the stomach. The principle of the breath test is shown in Figure 1.
Figure 1: Principle of Breath Test

To detect H.pylori, urea labeled with 14C is swallowed by the patient. If gastric urease from H.pylori is present, urea is split to form CO2 and NH3 at the interface between the gastric epithelium and lumen and 14CO2 is absorbed into the blood and exhaled in the breath.
Following ingestion of the capsule by a patient with H.pylori, 14CO2 excretion in the breath peaks between 10 and 15 minutes and declines thereafter with a biological half-life of about 15 minutes. 14C-urea that is not hydrolyzed by H.pylori is excreted in the urine with a half-life of approximately 12 hours. About 10% of the 14C remains in the body at 72 hours and is gradually excreted with a biological half-life of 40 days.
Clinical Studies
Two studies were performed. In both studies, patients with gastrointestinal
symptoms underwent the breath test and an endoscopy. During the
endoscopy, biopsy samples were taken from the antral gastric mucosa
for histological analysis (2 samples, Giemsa stain) and rapid urease test
(1 sample, CLOtest
Results were reported as disintegrations per minute (DPM). Analysis for accuracy used the ten minute breath sample. A breath sample DPM <50 was defined as a negative result. DPM ≥200 was defined as a positive result. DPM in the range of 50 -199 was classified as indeterminate.
STUDY 1
Of 186 patients who had histopathology and CLOtest

Histology and CLOtest |
|||||
|---|---|---|---|---|---|
| H.pylori | Positive | Negative | Total | ||
Notes:
PYtest  |
|||||
|
ppv = positive predictive value (true positive divided by total PYtest  |
|||||
|
npv = negative predictive value (true negative divided by total PYtest  |
|||||
PYtest |
Positive | 51 | 8 | 59 | ppv. 86% |
| (DPM | Indeterminate | 1 | 8 | 9 | |
| 10m.) | Negative | 1 | 117 | 118 | npv. 99% |
| Total | 53 | 133 | 186 | ||
| sensitivity 96% |
specificity 88% |
||||
STUDY 2
Breath tests were performed on 436 outpatients attending
gastroenterology practices at sites in the United States. Seventy-six patients (40 men, 36 women) who had histology and CLOtest
Histology and CLOtest |
|||||
|---|---|---|---|---|---|
| H.pylori | Positive | Negative | Total | ||
Notes:
PYtest |
|||||
|
ppv = positive predictive value (true positive divided by total PYtest  |
|||||
|
npv = negative predictive value (true negative divided by total PYtest  |
|||||
PYtest |
Positive | 22 | 0 | 22 | ppv. 100% |
| (DPM | Indeterminate | 4 | 2 | 6 | |
| 10m.) | Negative | 1 | 47 | 48 | npv. 98% |
| Total | 27 | 49 | 76 | ||
| sensitivity 82% |
specificity 96% |
||||
PYtest Indications and Usage
PYtest
Contraindications
None
Warnings
None
Precautions
General
After the patient ingests the 14C-urea capsule, the sample collected for test purposes is for in vitro diagnostic use only.
A false positive test could occur in patients who have achlorhydria. Very rarely, a false positive test may occur due to urease associated with Helicobacters other than H.pylori (i.e. Helicobacter heilmanni).
Limitations of the Test
- The test has been evaluated in outpatients before elective endoscopy.
- Test results should be evaluated with clinical signs and patient history when diagnosing H.pylori infection.
- The performance characteristics of the test have not been established for monitoring the efficacy of antimicrobial therapies for the treatment of H.pylori infection.
- A negative result does not completely rule out the possibility of
H.pylori infection. If clinical signs and patient history suggest H.pylori
infection, repeat the PYtest

Registered Trademark or Trademark of Kimberly-Clark Worldwide, Inc. or use an alternative diagnostic method. - The integrity of samples in balloons sent by air transport has not been adequately determined. In studies simulating the effects of air transport for two to seven days at temperatures of -40°C, 20°C and 55°C, no balloon failures were observed. However, the data could not provide statistical determination that no changes in 14CO2 concentration took place.
- For ground transport, integrity of samples in balloons has not been determined beyond 7 days. During this time frame, concentration of labeled CO2 can decrease as much as 0.36% per day.
Radioactivity
Persons concerned about very low doses of radioactivity may postpone the test or may decide to use an alternative means of diagnosis. The test produces radiation exposure equal to 24 hours of normal background. In animal experiments, such low doses of radiation do not carry measurable risk.
Preclinical studies were not conducted on 14C-urea. The estimated
dose equivalent received from a single administration of PYtest
Information for Patients
It is necessary for the patient to fast for 6 hours before the test. The patient should also be off antibiotics and bismuth for 1 month, and proton pump inhibitors and sucralfate for 2 weeks prior to the test. Instruct the patient not to handle the capsule directly as this may interfere with the test result. The capsule should be swallowed intact. Do not chew the capsule.
Carcinogenesis, mutagenesis, impairment of fertility
No studies have been conducted with 14C-urea to evaluate its potential for carcinogenicity, impairment of fertility, or mutagenicity.
Drug Interactions
Antibiotics, proton pump inhibitors, sucralfate, and bismuth preparations are known to suppress H.pylori. Ingestion of antibiotics or bismuth within 4 weeks and proton pump inhibitors or sucralfate within 2 weeks prior to performing the test may give false negative results.
Pregnancy
Pregnancy category C
Animal reproduction studies have
not been conducted with PYtest


Nursing mothers
It is not known whether this drug is excreted in
human milk. Because many drugs are excreted in human milk, caution
should be exercised when PYtest
Pediatric Use
Clinical studies in children have not been conducted.
However, PYtest
Side Effects
No adverse reactions were reported in clinical trials.
Overdosage
Risk from radiation is negligible even with a 1000 capsule overdose (0.3 rem). If overdose occurs, the patient may drink one glass of water (150 mL) every hour to hasten excretion of the isotope. Maximum excretion of Urea is achieved at a urine output of ≥2.0 mL/min.
Dosage and Administration
Materials provided
As shown in Figure 2, the PYtest
- PYtest

Registered Trademark or Trademark of Kimberly-Clark Worldwide, Inc. capsule - Two 30 mL disposable cups
- One drinking straw
- One mylar collection balloon
- One report form
- One mailing box with labels
The kit includes analysis by Kimberly-Clark of one balloon from one patient at one time point.
Figure 2: PYtest
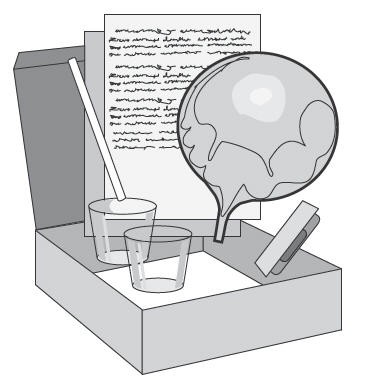
Materials Needed but not Provided
- Stopwatch/Timer capable of timing an interval up to 10 minutes.
- Water (40mL)
Dosage
One PYtest
Procedural Notes
- Inform the patient to fast for 6 hours prior to the test.
- The patient should be off antibiotics and bismuth for 1 month, and proton pump inhibitors and sucralfate for 2 weeks prior to the test.
- Have patient sitting at rest while doing the test.
- The capsule should not be handled directly as this may interfere with the test result.
- To avoid contamination by bacteria in the mouth, the capsule should be swallowed intact. Do not chew capsule.
Step by Step Procedure for Balloon
| Before the test |
|
| Minus 1 |
|
| 0 minute |
|
| 3 minutes | Ask the patient to drink another 20 mL of lukewarm water (in case the capsule may have lodged in the esophagus and not yet reached the gastric mucosa). |
| 10 minutes |
|
| After the test | Place the filled balloon and breath test report in the box and forward to Kimberly-Clark for analysis. |
Quality Control
A minimum of 1 mM of CO2 is required to perform analysis of a breath sample. The amount of breath required to provide 1 mM of CO2 varies depending on the amount of CO2 the patient is producing. Since a full balloon typically contains at least 1 mM of CO2, the balloon should be completely filled.
Results
| Interpretation of results (10 minute sample) | |
| <50 DPM | Negative for H.pylori |
| 50-199 DPM | Indeterminate for H.pylori |
| ≥200 DPM | Positive for H.pylori |
The indeterminate result should be evaluated by repeating the PYtest

The cutoff point of 50 DPM was determined to be the mean + 3SD of results obtained in patients who did not have H.pylori.
DPM = Disintegrations per minute
| Factor | Result | Comment |
|---|---|---|
| Recent antibiotic or bismuth (Pepto-Bismol, etc.) | false neg. | Relapse of partially treated Hp may take 1-4 weeks. |
| Omeprazole (or other proton pump inhibitors) | false neg. | These agents suppress Hp in 40% of patients. Discontinue for at least 2 weeks before performing the PYtest |
| Resective gastric surgery | false neg. | Isotope may empty rapidly from the stomach. |
| Resective gastric surgery | false pos. | Patient may be achlorhydric and have bacterial overgrowth (non-Hp urease). |
| Food in stomach (also bezoar, gastroparesis) | unknown | Isotope may not come into contact with gastric mucosa. Patient may be achlorhydric and/or have bacterial overgrowth (non-Hp urease). |
Expected Values
As shown in Figure 3 approximately 30% of patients tested will be positive for H.pylori.
Figure 3: Histogram showing DPM distribution for the PYtest

Note: DPM groupings were calculated on a logarithmic scale. Empty DPM groupings were not included. Chart includes all patients from Studies 1 and 2.
Frequency of DPM group includes samples with DPM < Group Name.
DPM = Disintegrations per minute
Gold Standard = Agreement between histology and CLOtest
If the capsule is damaged or appears abnormal in any way, it may give inaccurate results.
How Supplied
PYtest

PYtest
The PYtest
PYtest
Rx Only
Kimberly-Clark Distributed in the U.S. by Kimberly-Clark Global Sales, LLC,
Roswell, GA 30076 USA
In USA, please call 1-800-KCHELPS • www.kchealthcare.com
Kimberly-Clark, Roswell, GA 30076 USA
Kimberly-Clark N.V., Belgicastraat 13, 1930 Zaventem, Belgium
Sponsored in Australia by Kimberly-Clark Australia Pty Limited; 52 Alfred Street,
Milsons Point, NSW 2061 • 1-800-101-021
©2003 KCWW. All rights reserved.
14-63-136-0-00/70080910 12/07
PRINCIPAL DISPLAY PANEL - PYtest* Kit Label
Kimberly-Clark* PYtest* Kit
14C-Urea Breath Test
for the detection of Helicobacter pylori
Contents - 10 PYtest* Capsules
PYtest* Breath Collection Accessories
For Dosage Information, Please See Package Insert
1 PYtest* capsule contains 1 µCi 14C-Urea
14C-Urea (5730years1/2, 156keV[max] β-emission)
For In Vitro Diagnostic Use
Rx only
Store at 15°- 30°C (59°- 86°F)
*Registered Trademark or Trademark of Kimberly-Clark Worldwide, Inc.
©2003 KCWW. All rights reserved. 20-63-141-0-00/70080967

PYtestUrea, C-14 CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||