RANITIDINE
Ranitidine Syrup (Ranitidine Oral Solution, USP)
FULL PRESCRIBING INFORMATION: CONTENTS*
- RANITIDINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- RANITIDINE INDICATIONS AND USAGE
- RANITIDINE CONTRAINDICATIONS
- PRECAUTIONS
- RANITIDINE ADVERSE REACTIONS
- OVERDOSAGE
- RANITIDINE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
RANITIDINE DESCRIPTION
2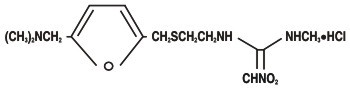
132243
CLINICAL PHARMACOLOGY
2Pharmacokinetics:
Absorption:
Distribution:
Metabolism:
Excretion:
Geriatrics: PRECAUTIONS: Geriatric Use DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients With Impaired Renal Function)
Pediatrics: 1/2maxmax
| Population (age) |
n |
Dosage Form (dose) |
Cmax
(ng/mL) |
Tmax
(hours) |
| Gastric or duodenal ulcer (3.5 to 16 years) |
12 |
Tablets (1 to 2 mg/kg) |
54 to 492 |
2.0 |
| Otherwise healthy requiring ranitidine (0.7 to 14 years, Single dose) |
10 |
Oral Solution (2 mg/kg) |
244 |
1.61 |
| Otherwise healthy requiring ranitidine (0.7 to 14 years, Multiple dose) |
10 |
Oral Solution (2 mg/kg) |
320 |
1.66 |
Pharmacodynamics:
Antisecretory Activity: 1. Effects on Acid Secretion:
| Time After Dose, h |
% Inhibition of Gastric Acid Output by Dose, mg |
||||
| 75 - 80 |
100 |
150 |
200 |
||
| Basal |
Up to 4 |
99 |
95 |
||
| Nocturnal |
Up to 13 |
95 |
96 |
92 |
|
| Betazole |
Up to 3 |
97 |
99 |
||
| Pentagastrin |
Up to 5 |
58 |
72 |
72 |
80 |
| Meal |
Up to 3 |
73 |
79 |
95 |
|
2. Effects on Other Gastrointestinal Secretions:
Pepsin:
Intrinsic Factor:
Serum Gastrin:
Other Pharmacologic Actions:
- Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
- Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
- Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
- No change in cortisol, aldosterone, androgen, or estrogen levels.
- No antiandrogenic action.
- No effect on count, motility, or morphology of sperm.
Clinical Trials: Active Duodenal Ulcer:
| * All patients were permitted p.r.n. antacids for relief of pain. † P<0.0001. |
||||
| Ranitidine* |
Placebo* |
|||
| Number Entered |
Healed/ Evaluable |
Number Entered |
Healed/ Evaluable |
|
| Outpatients |
195 |
69/182 (38%)† |
188 |
31/164 (19%) |
| Week 2 |
||||
| Week 4 |
137/187 (73%)† |
76/168 (45%) |
||
| |
Ulcer Healed |
Ulcer Not Healed |
| Ranitidine |
0.06 |
0.71 |
| Placebo |
0.71 |
1.43 |
Maintenance Therapy in Duodenal Ulcer:
| % = Life table estimate. * = P<0.05 (ranitidine versus comparator). RAN = ranitidine. PLC = placebo. |
|||||
| Double-Blind, Multicenter, Placebo-Controlled Trials |
|||||
| Multicenter Trial |
Drug |
Duodenal Ulcer Prevalence |
No. of Patients |
||
| 0 - 4 Months |
0 - 8 Months |
0 - 12 Months |
|||
| USA |
RAN |
20%* |
24%* |
35%* |
138 |
| PLC |
44% |
54% |
59% |
139 |
|
| Foreign |
RAN |
12%* |
21%* |
28%* |
174 |
| PLC |
56% |
64% |
68% |
165 |
|
Gastric Ulcer:
| * All patients were permitted p.r.n. antacids for relief of pain. † P = 0.009. |
||||
| |
Ranitidine* |
Placebo* |
||
| Number Entered |
Healed/ Evaluable |
Number Entered |
Healed/ Evaluable |
|
| Outpatients |
92 |
16/83 (19%) |
94 |
10/83 (12%) |
| Week 2 |
||||
| Week 6 |
50/73 (68%)† |
35/69 (51%) |
||
Maintenance of Healing of Gastric Ulcers:
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
Gastroesophageal Reflux Disease (GERD):
Erosive Esophagitis:
| * All patients were permitted p.r.n. antacids for relief of pain. † P<0.001 versus placebo. |
||
| |
Healed/Evaluable |
|
| Placebo* n = 229 |
Ranitidine 150 mg q.i.d.* n = 215 |
|
| Week 4 |
43/198 (22%) |
96/206 (47%)†
|
| Week 8 |
63/176 (36%) |
142/200 (71%)†
|
| Week 12 |
92/159 (58%) |
162/192 (84%)†
|
Maintenance of Healing of Erosive Esophagitis:
RANITIDINE INDICATIONS AND USAGE
- Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
- Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year.
- The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
- Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
- Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
- Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with ranitidine 150 mg b.i.d.
- Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with ranitidine 150 mg q.i.d.
- Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
RANITIDINE CONTRAINDICATIONS
PRECAUTIONS
General:
- Symptomatic response to therapy with ranitidine does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (see DOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Drug Interactions:
Procainamide:
Warfarin:
Atazanavir:
Delavirdine: 2
Gefitinib:
Glipizide:
Ketoconazole:
Midazolam:
Triazolam:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Salmonella, Escherichia coli
Pregnancy: Teratogenic Effects:
Nursing Mothers:
Pediatric Use:
Geriatric Use:
RANITIDINE ADVERSE REACTIONS
The following have been reported as events in clinical trials or in the routine management of patients treated with ranitidine. The relationship to therapy with ranitidine has been unclear in many cases. Headache, sometimes severe, seems to be related to administration of ranitidine.
Central Nervous System: Rarely, malaise, dizziness, somnolence, insomnia, and vertigo. Rare cases of reversible mental confusion, agitation, depression, and hallucinations have been reported, predominantly in severely ill elderly patients. Rare cases of reversible blurred vision suggestive of a change in accommodation have been reported. Rare reports of reversible involuntary motor disturbances have been received.
Cardiovascular: As with other H2-blockers, rare reports of arrhythmias such as tachycardia, bradycardia, atrioventricular block, and premature ventricular beats.
Gastrointestinal: Constipation, diarrhea, nausea/vomiting, abdominal discomfort/pain, and rare reports of pancreatitis.
Hepatic: There have been occasional reports of hepatocellular, cholestatic, or mixed hepatitis, with or without jaundice. In such circumstances, ranitidine should be immediately discontinued. These events are usually reversible, but in rare circumstances death has occurred. Rare cases of hepatic failure have also been reported. In normal volunteers, SGPT values were increased to at least twice the pretreatment levels in 6 of 12 subjects receiving 100 mg q.i.d. intravenously for 7 days, and in 4 of 24 subjects receiving 50 mg q.i.d. intravenously for 5 days.
Musculoskeletal: Rare reports of arthralgias and myalgias.
Hematologic: Blood count changes (leukopenia, granulocytopenia, and thrombocytopenia) have occurred in a few patients. These were usually reversible. Rare cases of agranulocytosis, pancytopenia, sometimes with marrow hypoplasia, and aplastic anemia and exceedingly rare cases of acquired immune hemolytic anemia have been reported.
Endocrine: Controlled studies in animals and man have shown no stimulation of any pituitary hormone by ranitidine and no antiandrogenic activity, and cimetidine-induced gynecomastia and impotence in hypersecretory patients have resolved when ranitidine has been substituted. However, occasional cases of impotence and loss of libido have been reported in male patients receiving ranitidine, but the incidence did not differ from that in the general population.
Integumentary: Rash, including rare cases of erythema multiforme. Rare cases of alopecia and vasculitis.
Respiratory: A large epidemiological study suggested an increased risk of developing pneumonia in current users of histamine-2-receptor antagonists (H2RAs) compared to patients who had stopped H2RA treatment, with an observed adjusted relative risk of 1.63 (95% CI, 1.07-2.48). However, a causal relationship between use of H2RAs and pneumonia has not been established.
Other: Rare cases of hypersensitivity reactions (e.g., bronchospasm, fever, rash, eosinophilia), anaphylaxis, angioneurotic edema, and small increases in serum creatinine.
OVERDOSAGE
50
DOSAGE AND ADMINISTRATION
Active Duodenal Ulcer: Active Duodenal UlcerMaintenance of Healing of Duodenal Ulcers:
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
Benign Gastric Ulcer:
Maintenance of Healing of Gastric Ulcers:
GERD:
Erosive Esophagitis:
Maintenance of Healing of Erosive Esophagitis:
Pediatric Use:
Treatment of Duodenal and Gastric Ulcers:
Maintenance of Healing of Duodenal and Gastric Ulcers:
Treatment of GERD and Erosive Esophagitis:
Dosage Adjustment for Patients With Impaired Renal Function:
HOW SUPPLIED
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Manufactured by:
Distributed by:

RANITIDINERANITIDINE HYDROCHLORIDE SYRUP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||