Ranitidine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- RANITIDINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- RANITIDINE HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- RANITIDINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
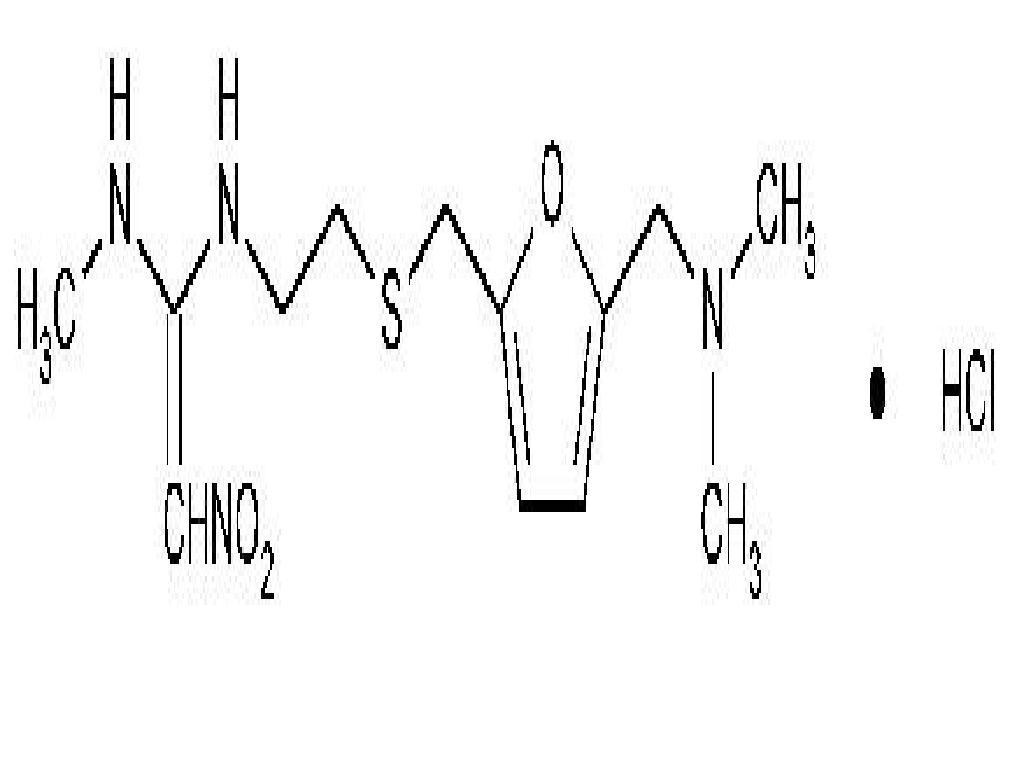
RANITIDINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
PRECAUTIONS: Geriatric UseDOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients with Impaired Renal Function
PRECAUTIONS: Pediatric UseDOSAGE AND ADMINISTRATION: Pediatric Us
INDICATIONS & USAGE
RANITIDINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONSPRECAUTIONS
DOSAGE AND ADMINISTRATION
LABORATORY TESTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY: PediatricsDOSADGE AND ADMINISTRATION: Pediatric UseCLINICAL PHARMACOLOGY: Pediatrics
GERIATRIC USE
CLINICAL PHARMACOLOGY: Pharmacokinetics: GeriatricsDOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients with Impaired Renal Function
RANITIDINE HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
ADVERSE REACTIONSDOSAGE & ADMINISTRATION
Clinical Trials: Active Duodenal UlcerCLINICAL PHARMACOLOGY: Pharmacokinetics
CLINICAL PHARMACOLOGY: Pharmacokinetics: GeriatricsPRECAUTIONS: Geriatric Use
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Ranitidine HydrochlorideRanitidine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!