Ranitidine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- RANITIDINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- RANITIDINE HYDROCHLORIDE INDICATIONS AND USAGE
- RANITIDINE HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- RANITIDINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- RANITIDINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
RANITIDINE HYDROCHLORIDE DESCRIPTION
2
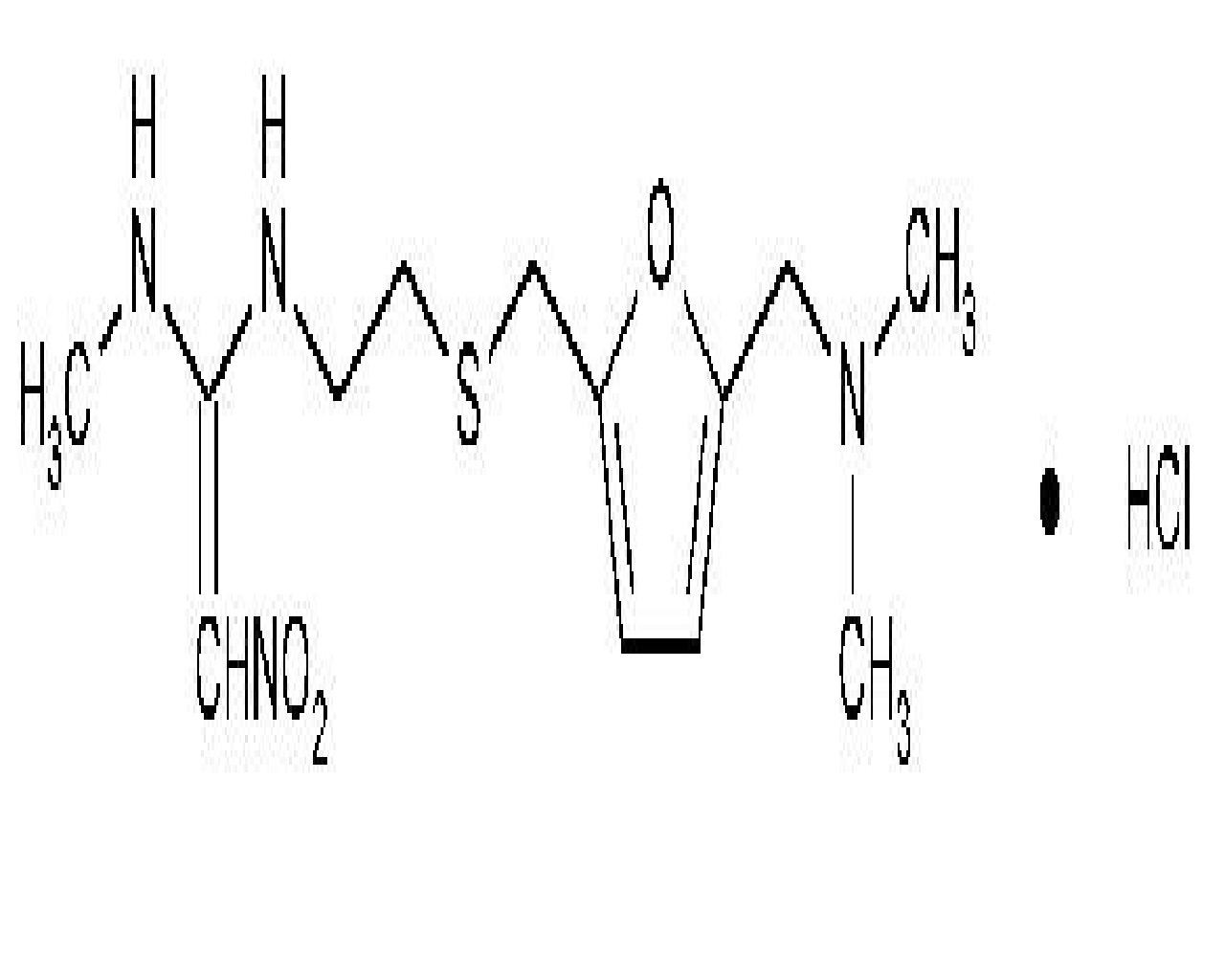
132243
CLINICAL PHARMACOLOGY
Ranitidine Tablets, USP is a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets, USP does not lower serum Ca++ in hypercalcemic states. Ranitidine Tablets, USP is not an anticholinergic agent.
Pharmacokinetics: Absorption: Ranitidine Tablets, USP is 50% absorbed after oral administration, compared to an intravenous (IV) injection with mean peak levels of 440 to 545 ng/mL occurring 2 to 3 hours after a 150-mg dose. Absorption is not significantly impaired by the administration of food or antacids. Propantheline slightly delays and increases peak blood levels of Ranitidine Tablets, USP, probably by delaying gastric emptying and transit time. In one study, simultaneous administration of high-potency antacid (150 mmol) in fasting subjects has been reported to decrease the absorption of Ranitidine Tablets, USP.
Distribution: The volume of distribution is about 1.4 L/kg. Serum protein binding averages 15%.
Metabolism: In humans, the N-oxide is the principal metabolite in the urine; however, this amounts to gretater then 4% of the dose. Other metabolites are the S-oxide (1%) and the desmethyl ranitidine (1%). The remainder of the administered dose is found in the stool. Studies in patients with hepatic dysfunction (compensated cirrhosis) indicate that there are minor, but clinically insignificant, alterations in ranitidine half-life, distribution, clearance, and bioavailability.
Excretion: The principal route of excretion is the urine, with approximately 30% of the orally administered dose collected in the urine as unchanged drug in 24 hours. Renal clearance is about 410 mL/min, indicating active tubular excretion. The elimination half-life is 2.5 to 3 hours. Four patients with clinically significant renal function impairment (creatinine clearance 25 to 35 mL/min) administered 50 mg of ranitidine intravenously had an average plasma half-life of 4.8 hours, a ranitidine clearance of 29 mL/min, and a volume of distribution of 1.76 L/kg. In general, these parameters appear to be altered in proportion to creatinine clearance (see DOSAGE AND ADMINISTRATION).
Geriatrics: The plasma half-life is prolonged and total clearance is reduced in the elderly population due to a decrease in renal function. The elimination half-life is 3 to 4 hours. Peak levels average 526 ng/mL following a 150-mg twice daily dose and occur in about 3 hours (see PRECAUTIONS: Geriatric Use and DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients with Impaired Renal Function).
Pediatrics: There are no significant differences in the pharmacokinetic parameter values for ranitidine in pediatric patients (from 1 month up to 16 years of age) and healthy adults when correction is made for body weight. The average bioavailability of ranitidine given orally to pediatric patients is 48% which is comparable to the bioavailability of ranitidine in the adult population. All other pharmacokinetic parameter values (t1/2 , Vd, and CL) are similar to those observed with intravenous ranitidine use in pediatric patients. Estimates of Cmax and Tmax are displayed in Table 1.
| Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing | ||||
| Population (age) | N | Dosage Form (dose) |
Cmax
(ng/mL |
Tmax
(hours) |
| Gastric or duodenal
ulcer (3.5 to 16 years) |
12 | Tablets| (1 to 2 mg/kg) |
54 to 492 | 2.0 |
| Otherwise healthy
requiring Ranitidine (0.7 to 14 years, Single dose) |
10 | Syrup (2 mg/kg) |
244 | 1.61 |
| Otherwise healthy
requiring Ranitidine (0.7 to 14 years, Multiple dose) |
10 | Syrup (2 mg/kg) |
320 | 1.66 |
Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION: Pediatric Use).
Pharmacodynamics: Serum concentrations necessary to inhibit 50% of stimulated gastric acid secretion are estimated to be 36 to 94 ng/mL. Following a single oral dose of 150 mg, serum concentrations of Ranitidine Tablets, USP are in this range up to 12 hours. However, blood levels bear no consistent relationship to dose or degree of acid inhibition.
Antisecretory Activity: 1. Effects on Acid Secretion: Ranitidine Tablets, USP inhibits both daytime and nocturnal basal gastric acid secretions as well as gastric acid secretion stimulated by food, betazole, and pentagastrin, as shown in Table 2.
| Table 2. Effect of Oral Ranitidine Tablets, USP on Gastric Acid Secretion | |||||
|
|
Time
After Dose, h |
% Inhibition of Gastric Acid Output by Dose, mg | |||
| 75-80 | 100 | 150 | 200 | ||
| Basal | Up to 4 |
|
99 | 95 |
|
| Nocturnal | Up to 13 | 95 | 96 | 92 |
|
| Betazole | Up to 3 |
|
97 | 99 |
|
| Pentagastin | Up to 5 | 58 | 72 | 72 | 80 |
| Meal | Up to 3 |
|
73 | 79 | 95 |
It appears that basal-, nocturnal-, and betazole-stimulated secretions are most sensitive to inhibition by Ranitidine Tablets, USP, responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are most difficult to suppress.
2. Effects on Other Gastrointestinal Secretions:
Pepsin: Oral Ranitidine Tablets, USP does not affect pepsin secretion. Total pepsin output is reduced in proportion to the decrease in volume of gastric juice.
Intrinsic Factor: Oral Ranitidine Tablets, USP has no significant effect on pentagastrin-stimulated intrinsic factor secretion.
Serum Gastrin: Ranitidine Tablets, USP has little or no effect on fasting or postprandial serum gastrin.
Other Pharmacologic Actions:
a. Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
b. Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
c. Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
d. No change in cortisol, aldosterone, androgen, or estrogen levels.
e. No antiandrogenic action.
f. No effect on count, motility, or morphology of sperm.
Pediatrics: Oral doses of 6 to 10 mg/kg per day in two or three divided doses maintain gastric pH less then 4 throughout most of the dosing interval.
Clinical Trials: Active Duodenal Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed duodenal ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets, USP as shown in Table 3
| Table 3. Duodenal Ulcer Patient Healing Rates | ||||
|
|
Ranitidine Tablets, USP | Placebo | ||
| Number Entered |
Healed /
Evaluable |
Number Entered |
Healed /
Evaluable |
|
| Outpatients | 195 | 69/182 (38%) † |
188 | 31-164 (19%) |
| Week 2 | ||||
| Week 4 | 137/187 (73%) † |
76/168 (45%) |
*All patients were permitted p.r.n. antacids for relief of pain.
†p greater then 0.0001.
In these studies, patients treated with Ranitidine Tablets, USP reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
| Table 4. Mean Daily Doses of Antacid | ||
|
|
Ulcer Healed | Ulcer Not Healed |
| Ranitidine | 0.06 | 0.71 |
| Placebo | 0.71 | 1.43 |
Foreign studies have shown that patients heal equally well with 150 mg b.i.d. and 300 mg h.s. (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg b.i.d. as compared to 300 mg h.s. (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
Maintenance Therapy in Duodenal Ulcer: Ranitidine has been found to be effective as maintenance therapy for patients following healing of acute duodenal ulcers. In 2 independent, double-blind, multicenter, controlled trials, the number of duodenal ulcers observed was significantly less in patients treated with Ranitidine Tablets, USP (150 mg h.s.) than in patients treated with placebo over a 12-month period.
| Table 5. Duodenal Ulcer Prevalence | |||||
| Double-Blind, Multicenter, Placebo-Controlled Trials | |||||
| Multicenter Trial |
Drug | Duodenal Ulcer Prevalence | No. Of Patients | ||
|
|
|
0-4
Months |
0-8
Months |
0-12
Months |
|
| USA | RAN | 20% | 24% | 35% | 138 |
| PLC | 44% | 54% | 59% | 139 | |
| Foreign | RAN | 12% | 21% | 28% | 174 |
| PLC | 56% | 64% | 68% | 165 |
% = Life table estimate.
* = P greater then 0.05 (Ranitidine Tablets, USP versus comparator).
RAN = ranitidine (Ranitidine Tablets, USP)
PLC = placebo.
As with other H2-antogonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
Gastric Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed gastric ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets, USP as shown in Table 6.
| Table 6. Gastric Ulcer Patient Healing Rates | ||||
|
|
Ranitidine Tablets, USP | Placebo | ||
| Number Entered |
Healed /
Evaluable |
Number Entered |
Healed /
Evaluable |
|
| Outpatients | 92 | 16/183 (19%) † |
94 | 10/83 (12%) |
| Week 2 | ||||
| Week 6 | 50/73 (68%) † |
35/69 (51%) |
*All patients were permitted p.r.n. antacids for relief of pain.
†P = 0.009.
In this multicenter trial, significantly more patients treated with Ranitidine Tablets, USP became pain free during therapy.
Maintenance of Healing of Gastric Ulcers: In two multicenter, double-blind, randomized, placebo-controlled, 12-month trials conducted in patients whose gastric ulcers had been previously healed, Ranitidine Tablets, USP 150 mg h.s. was significantly more effective than placebo in maintaining healing of gastric ulcers.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
Ranitidine Tablets, USP inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions (e.g., postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets, USP was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
Gastroesophageal Reflux Disease (GERD): In 2 multicenter, double-blind, placebo-controlled, 6-week trials performed in the United States and Europe, Ranitidine Tablets, USP 150 mg b.i.d. was more effective than placebo for the relief of heartburn and other symptoms associated with GERD. Ranitidine-treated patients consumed significantly less antacid than did placebo-treated patients.
The US trial indicated that Ranitidine Tablets, USP 150 mg b.i.d. significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect on heartburn extends through both the day and night time periods.
In 2 additional US multicenter, double-blind, placebo-controlled, 2-week trials, Ranitidine Tablets, USP 150 mg b.i.d. was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
Erosive Esophagitis: In two multicenter, double-blind, randomized, placebo-controlled, 12-week trials performed in the United States, Ranitidine Tablets, USP 150 mg q.i.d. was significantly more effective than placebo in healing endoscopically diagnosed erosive esophagitis and in relieving associated heartburn.
The erosive esophagitis healing rates were as follows:
| Table 7. Erosive Esophagitis Patient Healing Rates | ||
|
|
Healed / Evaluable | |
|
|
Placebo n=229 |
Ranitidine Tablets,
USP 150 mg q.i.d. n=215 |
| Week 4 | 43/198 (22%) | 96/206 (47%) † |
| Week 8 | 63/176 (36%) | 142/200 (71%) † |
| Week 12 | 92/159 (58%) | 162/192 (84%) † |
*All patients were permitted p.r.n. antacids for relief of pain.
†P greater then 0.001 versus placebo.
No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg q.i.d.
Maintenance of Healing of Erosive Esophagitis: In 2 multicenter, double-blind, randomized, placebo-controlled, 48-week trials conducted in patients whose erosive esophagitis had been previously healed, Ranitidine Tablets, USP 150 mg b.i.d. was significantly more effective than placebo in maintaining healing of erosive esophagitis.
RANITIDINE HYDROCHLORIDE INDICATIONS AND USAGE
RANITIDINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS
General:Laboratory Tests:
Drug Interactions:in vitro
Carcinogenesis, Mutagenesis, Impairment of Fertility:
(Salmonella, Escherichia coli)
Pregnancy:
Nursing Mothers:
Pediatric Use:
Geriatric Use:
RANITIDINE HYDROCHLORIDE ADVERSE REACTIONS
Central Nervous System:
Cardiovascular:2
Gastrointestinal:
Hepatic:
Musculoskeletal:
Hematologic:
Endocrine:
Integumentary:
Respiratory:222
Other:
OVERDOSAGE
50
RANITIDINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Active Duodenal Ulcer:Maintenance of Healing of Duodenal Ulcers:
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
Benign Gastric Ulcer:
Maintenance of Healing of Gastric Ulcers:
GERD:
Erosive Esophagitis:
Maintenance of Healing of Erosive Esophagitis:
Pediatric Use:
Treatment of Duodenal and Gastric Ulcers:
Maintenance of Healing of Duodenal and Gastric Ulcers:
Treatment of GERD and Erosive Esophagitis:
Dosage Adjustment for Patients With Impaired Renal Function:
HOW SUPPLIED
Amneal Pharmaceuticals of NY
Amneal Pharmaceuticals
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

Ranitidine HydrochlorideRanitidine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||