REFISSA
FULL PRESCRIBING INFORMATION: CONTENTS*
- REFISSA DESCRIPTION
- CLINICAL PHARMACOLOGY
- REFISSA INDICATIONS AND USAGE
- CLINICAL TRIALS DATA
- REFISSA CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- REFISSA ADVERSE REACTIONS
- OVERDOSAGE
- REFISSA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- MEDGUIDE SECTION
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - 40g carton label
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - 20g carton label
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - Obagi Medical 20g carton label
FULL PRESCRIBING INFORMATION
FOR TOPICAL USE ON THE FACE ONLY
REFISSA DESCRIPTION
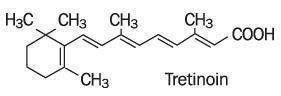
Refissa [Tretinoin Cream, USP (Emollient) 0.05%], contains the active ingredient tretinoin (a retinoid) in an emollient cream base. Tretinoin is a yellow-to-light-orange crystalline powder having a characteristic floral odor. Tretinoin is soluble in dimethylsulfoxide, slightly soluble in polyethylene glycol 400, octanol, and 100% ethanol. It is practically insoluble in water and mineral oil, and it is insoluble in glycerin. The chemical name for tretinoin is (all-E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclonexen-1-yl)-2,4,6,8-nonatetraenoic acid. Tretinoin is also referred to as all-trans-retinoic acid and has a molecular weight of 300.44. The structural formula is represented below.
Refissa contains tretinoin at a concentration of 0.05% w/w in a water-in-oil emulsion formulation consisting of light mineral oil, sorbitol solution, hydroxyoctacosanyl hydroxystearate; methoxy PEG-22/dodecyl glycol copolymer, PEG-45/dodecyl glycol copolymer, stearoxytrimethylsilane and stearyl alcohol, dimethicone 50 cs, methylparaben, edetate disodium, propylparaben, butylated hydroxytoluene, citric acid monohydrate, and purified water.
CLINICAL PHARMACOLOGY
The exact mechanism of action of tretinoin is unknown although retinoids are believed to exert an effect on the growth and differentiation of various epithelial cells. When applied topically, however, there was no noted increase in desmosine, hydroxyproline, or elastin mRNA in human skin. In addition, the role of the irritative nature of this product in effecting the positive effects attributed to this product for its indication has not yet been fully determined.
The transdermal absorption of tretinoin from various topical formulations ranged from 1% to 31% of applied dose, depending on whether it was applied to healthy skin or dermatitic skin. When percutaneous absorption of Tretinoin Cream, USP (Emollient) 0.05% was assessed in healthy male subjects (n=14) after a single application, as well as after repeated daily applications for 28 days, the absorption of tretinoin was less than 2% and endogenous concentrations of tretinoin and its major metabolites were unaltered.
REFISSA INDICATIONS AND USAGE
(To understand fully the indication for this product, please read the entire INDICATIONS AND USAGE section of the labeling.)
Refissa [Tretinoin Cream, USP (Emollient) 0.05%] is indicated as an adjunctive agent (see second bullet point below) for use in the mitigation (palliation) of fine wrinkles, mottled hyperpigmentation, and tactile roughness of facial skin in patients who do not achieve such palliation using comprehensive skin care and sun avoidance programs (see bullet 3 for populations in which effectiveness has not been established). REFISSA DOES NOT ELIMINATE WRINKLES, REPAIR SUN-DAMAGED SKIN, REVERSE PHOTOAGING, or RESTORE A MORE YOUTHFUL or YOUNGER DERMAL HISTOLOGIC PATTERN. Many patients achieve desired palliative effects on fine wrinkling, mottled hyperpigmentation, and tactile roughness of facial skin with the use of comprehensive skin care and sun avoidance programs including sunscreens, protective clothing, and emollient creams NOT containing tretinoin.
- Tretinoin Cream, USP (Emollient) 0.05% has demonstrated NO MITIGATING EFFECT on significant signs of chronic sun exposure such as coarse or deep wrinkling, skin yellowing, lentigines, telangiectasia, skin laxity, keratinocytic atypia, melanocytic atypia, or dermal elastosis.
- Refissa should be used under medical supervision as an adjunct to a comprehensive skin care and sun avoidance program that includes the use of effective sunscreens (minimum SPF of 15) and protective clothing when desired results on fine wrinkles, mottled hyperpigmentation, and roughness of facial skin have not been achieved with a comprehensive skin care and sun avoidance program alone.
- The effectiveness of Refissa [Tretinoin Cream, USP (Emollient) 0.05%] in the mitigation of fine wrinkles, mottled hyperpigmentation, and tactile roughness of facial skin has not been established in people greater than 50 years of age OR in people with moderately to heavily pigmented skin. In addition, patients with visible actinic keratoses and patients with a history of skin cancer were excluded from clinical trials of Tretinoin Cream, USP (Emollient) 0.05%. Thus the effectiveness and safety of Refissa in these populations are not known at this time.
- Neither the safety nor the effectiveness of Tretinoin Cream, USP (Emollient) for the prevention or treatment of actinic keratoses or skin neoplasms has been established.
- Neither the safety nor the efficacy of using Tretinoin Cream, USP (Emollient) 0.05% daily for greater than 48 weeks has been established, and daily use beyond 48 weeks has not been systematically and histologically investigated in adequate and well-controlled trials. (See WARNINGS section.)
CLINICAL TRIALS DATA
Two adequate and well-controlled trials were conducted involving a total of 161 evaluable patients (under 50 years of age) treated with Tretinoin Cream, USP (Emollient) 0.05% and 154 evaluable patients treated with the vehicle emollient cream on the face for 24 weeks as an adjunct to a comprehensive skin care and sun avoidance program, to assess the effects on fine wrinkling, mottled hyperpigmentation, and tactile skin roughness. Patients were evaluated at baseline on a 10-point scale, and changes from that baseline rating were categorized as follows:
No Improvement: No change or an increase of 1 unit or more
Minimal Improvement: Reduction of 1 unit
Moderate Improvement: Reduction of 2 units or more
In these trials, the fine wrinkles, mottled hyperpigmentation, and tactile roughness of the facial skin were thought to be caused by multiple factors which included intrinsic aging or environmental factors, such as chronic sun exposure.
The results of these assessments are as follows:
| *CSP= Comprehensive skin protection and sun avoidance programs included use of sunscreens, protective clothing, and emollient cream. | |||
| FINE WRINKLING | |||
|
NO IMPROVEMENT |
MINIMAL IMPROVEMENT |
MODERATE IMPROVEMENT |
|
| Tretinoin Cream, USP (Emollient) 0.05% + CSP* | 36% | 40% | 24% |
| Vehicle + CSP | 62% | 30% | 8% |
| MOTTLED HYPERPIGMENTATION | |||
|
NO IMPROVEMENT |
MINIMAL IMPROVEMENT |
MODERATE IMPROVEMENT |
|
| Tretinoin Cream, USP (Emollient) 0.05% + CSP* | 35% | 27% | 38% |
| Vehicle + CSP | 53% | 21% | 27% |
| TACTILE SKIN ROUGHNESS | |||
|
NO IMPROVEMENT |
MINIMAL IMPROVEMENT |
MODERATE IMPROVEMENT |
|
| Tretinoin Cream, USP (Emollient) 0.05% + CSP* | 49% | 35% | 16% |
| Vehicle + CSP | 67% | 23% | 10% |
Most of the improvement in these signs was noted during the first 24 weeks of therapy. Thereafter, therapy primarily maintained the improvement realized during the first 24 weeks.
A majority of patients will lose most mitigating effects of Refissa on fine wrinkles, mottled hyperpigmentation, and tactile roughness of facial skin with discontinuation of a comprehensive skin care and sun avoidance program including Refissa; however, the safety and effectiveness of using Refissa daily for greater than 48 weeks have not been established.
REFISSA CONTRAINDICATIONS
This drug is contraindicated in individuals with a history of sensitivity reactions to any of its components. It should be discontinued if hypersensitivity to any of its ingredients is noted.
WARNINGS
- Refissa [Tretinoin Cream, USP (Emollient) 0.05%] is a dermal irritant, and the results of continued irritation of the skin for greater than 48 weeks in chronic long-term use are not known. There is evidence of atypical changes in melanocytes and keratinocytes, and of increased dermal elastosis in some patients treated with Tretinoin Cream, USP (Emollient) 0.05% for longer than 48 weeks. The significance of these findings is unknown.
- Safety and effectiveness of Refissa in individuals with moderately or heavily pigmented skin have not been established.
- Refissa should not be administered if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the possibility of augmented phototoxicity.
Because of heightened burning susceptibility, exposure to sunlight (including sunlamps) should be avoided or minimized during use of Refissa. Patients must be warned to use sunscreens (minimum SPF of 15) and protective clothing when using Refissa. Patients with sunburn should be advised not to use Refissa until fully recovered. Patients who may have considerable sun exposure, e.g., due to their occupation and those patients with inherent sensitivity to sunlight, should exercise particular caution when using Refissa and assure that the precautions outlined in the Patient Package Insert are observed.
Tretinoin should be kept out of the eyes, mouth, angles of the nose, and mucous membranes. Topical use may cause severe local erythema, pruritus, burning, stinging, and peeling at the site of application. If the degree of local irritation warrants, patients should be directed to use less medication, decrease the frequency of application, discontinue use temporarily, or discontinue use altogether.
Tretinoin has been reported to cause severe irritation on eczematous skin and should be used only with caution in patients with this condition.
Application of larger amounts of medication than recommended will not lead to more rapid or better results, and marked redness, peeling, or discomfort may occur.
PRECAUTIONS
General
Refissa [Tretinoin Cream, USP (Emollient) 0.05%] should only be used as an adjunct to a comprehensive skin care and sun avoidance program. (See INDICATIONS AND USAGE section.)
If a drug sensitivity, chemical irritation, or a systemic adverse reaction develops, use of Refissa should be discontinued.
Weather extremes, such as wind or cold, may be more irritating to patients using Refissa.
Information for Patients
See Patient Package Insert.
Drug Interactions
Concomitant topical medications, medicated or abrasive soaps, shampoos, cleansers, cosmetics with a strong drying effect, products with high concentrations of alcohol, astringents, spices or lime, permanent wave solutions, electrolysis, hair depilatories or waxes, and products that may irritate the skin should be used with caution in patients being treated with Refissa [Tretinoin Cream, USP (Emollient) 0.05%] because they may increase irritation with Refissa.
Refissa should not be administered if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the possibility of augmented phototoxicity.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a lifetime dermal study in CD-1 mice at 100 and 200 times the average recommended human topical clinical dose, a few skin tumors in the female mice and liver tumors in male mice were observed. The biological significance of these findings is not clear because they occurred at doses that exceeded the dermal maximally tolerated dose (MTD) of tretinoin and because they were within the background natural occurrence rate for these tumors in this strain of mice. There was no evidence of carcinogenic potential when tretinoin was administered topically at a dose 5 times the average recommended human topical clinical dose. For purposes of comparisons of the animal exposure to the human exposure, the “recommended human topical clinical dose” is defined as 500 mg of Refissa applied daily to a 50 kg person.
In a chronic, two-year bioassay of Vitamin A acid in mice performed by Tsubura and Yamamoto, generalized amyloid deposition was reported in all groups in the basal layer of the Vitamin A treated skin. In CD-1 mice, a similar study reported hyalinization of the treated skin sites and the incidence of this finding was 0/50, 3/50, and 2/50 in male mice and 1/50, 0/50, 4/50 and 2/50 in female mice from the vehicle control, 0.25 mg/kg, 0.5 mg/kg, and 1 mg/kg groups, respectively.
Studies in hairless albino mice suggest that tretinoin may enhance the tumorigenic potential of carcinogenic doses of UVB and UVA light from a solar simulator. In other studies, when lightly pigmented hairless mice treated with tretinoin were exposed to carcinogenic doses of UVB light, the incidence and rate of development of skin tumors were either reduced or no effect was seen. Due to significantly different experimental conditions, no strict comparison of these disparate data is possible at this time. Although the significance of these studies in humans is not clear, patients should minimize exposure to sun.
The mutagenic potential of tretinoin was evaluated in the Ames assay and in the in vivo mouse micronucleus assay, both of which were negative.
Dermal Segment l and III studies with Tretinoin Cream, USP (Emollient) 0.05% have not been performed in any species. In oral Segment I and Segment III studies in rats with tretinoin, decreased survival of neonates and growth retardation were observed at doses in excess of 2 mg/kg/day (>400 times the average human topical clinical dose).
Pregnancy
ORAL tretinoin has been shown to be teratogenic in rats, mice, rabbits, hamsters, and subhuman primates. It was teratogenic and fetotoxic in rats when given orally or topically in doses 1000 times the average recommended human topical clinical dose. However, variations in teratogenic doses among various strains of rats have been reported. In the cynomolgus monkey, which, metabolically, is closer to humans for tretinoin than the other species examined, fetal malformations were reported at doses of 10 mg/kg/day or greater, but none were observed at 5 mg/kg/day (1000 times the average recommended human topical clinical dose), although increased skeletal variations were observed at all doses. A dose-related increase in embryolethality and abortion was reported. Similar results have also been reported in pigtail macaques.
TOPICAL tretinoin in animal teratogenicity tests has generated equivocal results. There is evidence for teratogenicity (shortened or kinked tail) of topical tretinoin in Wistar rats at doses greater than 1 mg/kg/day (200 times the recommended human topical clinical dose). Anomalies (humerus: short 13%, bent 6%, os parietal incompletely ossified 14%) have also been reported when 10 mg/kg/day was dermally applied.
There are other reports in New Zealand White rabbits administered with doses of approximately 80 times the recommended human topical clinical dose of an increased incidence of domed head and hydrocephaly, typical of retinoid-induced fetal malformations in this species.
In contrast, several well-controlled animal studies have shown that dermally applied tretinoin was not teratogenic, at doses of 100 and 200 times the recommended human topical clinical dose, in rats and rabbits, respectively.
With widespread use of any drug, a small number of birth defect reports associated temporally with the administration of the drug would be expected by chance alone. Thirty cases of temporally-associated congenital malformations have been reported during two decades of clinical use of another formulation of topical tretinoin (Retin-A). Although no definite pattern of teratogenicity and no causal association has been established from these cases, 5 of the reports describe the rare birth defect category holoprosencephaly (defects associated with incomplete midline development of the forebrain). The significance of these spontaneous reports in terms of risk to the fetus is not known.
Dermal tretinoin has been shown to be fetotoxic in rabbits when administered in doses 100 times the recommended topical human clinical dose. Oral tretinoin has been shown to be fetotoxic in rats when administered in doses 500 times the recommended topical human clinical dose.
There are, however, no adequate and well-controlled studies in pregnant women. Refissa [Tretinoin Cream, USP (Emollient) 0.05%] should not be used during pregnancy.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Refissa is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in patients less than 18 years of age have not been established.
Geriatric Use
Safety and effectiveness in individuals older than 50 years of age have not been established.
REFISSA ADVERSE REACTIONS
(See WARNINGS and PRECAUTIONS sections.)
In double-blind, vehicle-controlled studies involving 179 patients who applied Tretinoin Cream, USP (Emollient) 0.05% to their faces, adverse reactions associated with the use of Tretinoin Cream, USP (Emollient) 0.05% were limited primarily to the skin. During these trials, 4% of patients had to discontinue use of Tretinoin Cream, USP (Emollient) 0.05% because of adverse reactions. These discontinuations were due to skin irritation or related cutaneous adverse reactions.
Local reactions such as peeling, dry skin, burning, stinging, erythema, and pruritus were reported by almost all subjects during therapy with Tretinoin Cream, USP (Emollient) 0.05%. These signs and symptoms were usually of mild to moderate severity and generally occurred early in therapy. In most patients the dryness, peeling, and redness recurred after an initial (24 week) decline.
OVERDOSAGE
Application of larger amounts of medication than recommended has not been shown to lead to more rapid or better results, and marked redness, peeling, or discomfort may occur. Oral ingestion of the drug may lead to the same side effects as those associated with excessive oral intake of Vitamin A.
REFISSA DOSAGE AND ADMINISTRATION
- Do NOT use Refissa [Tretinoin Cream, USP (Emollient) 0.05%] if the patient is pregnant or is attempting to become pregnant or is at high risk of pregnancy
- Do NOT use Refissa if the patient is sunburned or if the patient has eczema or other chronic skin condition(s)
- Do NOT use Refissa if the patient is inherently sensitive to sunlight
- Do NOT use Refissa if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the possibility of augmented phototoxicity.
Patients require detailed instruction to obtain maximal benefits and to understand all the precautions necessary to use this product with greatest safety. The physician should review the Patient Package Insert.
Refissa should be applied to the face once a day before retiring, using only enough to cover the entire affected area lightly. Patients should gently wash their faces with a mild soap, pat the skin dry, and wait 20 to 30 minutes before applying Refissa. The patient should apply a pea-sized amount of cream to cover the entire face lightly. Special caution should be taken when applying the cream to avoid the eyes, ears, nostrils, and mouth.
Application of Refissa [Tretinoin Cream, USP (Emollient) 0.05%] may cause a transitory feeling of warmth or slight stinging.
Mitigation (palliation) of facial fine wrinkling, mottled hyperpigmentation, and tactile roughness may occur gradually over the course of therapy. Up to six months of therapy may be required before the effects are seen. Most of the improvement noted with Refissa is seen during the first 24 weeks of therapy. Thereafter, therapy primarily maintains the improvement realized during the first 24 weeks.
With discontinuation of Refissa therapy, a majority of patients will lose most mitigating effects of Refissa on fine wrinkles, mottled hyperpigmentation, and tactile roughness of facial skin; however, the safety and effectiveness of using Refissa daily for greater than 48 weeks have not been established.
Application of larger amounts of medication than recommended may not lead to more rapid results or better results, and marked redness, peeling, or discomfort may occur.
Patients treated with Refissa may use cosmetics, but the areas to be treated should be cleansed thoroughly before the medication is applied. (See PRECAUTIONS section.)
HOW SUPPLIED
Refissa is available in the following:
40 g tubes NDC 50224-002-03
20 g tubes NDC 50224-002-06
20 g tubes NDC 50224-002-02 Obagi Medical
Storage: Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]. DO NOT FREEZE.
Rx only.
Printed in USA
Manufactured by DPT Laboratories, San Antonio, TX 78215
Distributed by Suneva Medical Inc., San Diego, CA 92121
MEDGUIDE SECTION
[Tretinoin Cream, USP (Emollient) 0.05%]
FOR TOPICAL USE ON THE FACE ONLY
What is the Most Important Information about Refissa [Tretinoin Cream, USP (Emollient) 0.05%]
Refissa is a serious medication. It does not eliminate wrinkles or repair sundamaged skin. It may help treat fine wrinkles, spotty discoloration, and rough feeling skin, but it does not “cure” these conditions. Refissa should only be used under supervision of your health care provider as part of a broad skin care program. This program should include avoiding direct sunlight (by using protective clothing and sunscreens with a minimum SPF of 15) and using other moisturizing facial creams that do not contain tretinoin.
You should use Refissa only at bedtime. Do not use drying skin care products. Use the smallest amount of Refissa needed and avoid getting it in your eyes, ears, nose or mouth.
WARNING: Do not use Refissa if you are pregnant or attempting to become pregnant. Avoid sunlight and any other medicines that may increase your sensitivity to sunlight (see below).
Refissa has not been studied in people who are over 50 years of age or in people with moderately or darkly pigmented skin.
What is Refissa?
(What can I expect from Refissa?)
Refissa is a serious medication that may help treat but will not “cure” fine wrinkles, spotty skin discoloration, and rough feeling skin.
Studies show that after 24 weeks, about 30% of the people who used Tretinoin Cream, USP (Emollient) 0.05% for fine wrinkles or spotty discoloration had moderate improvement, another 35% had minimal improvement and 35% had no improvement. About 16% of the people who used Tretinoin Cream, USP (Emollient) 0.05% for rough skin had moderate improvement, 35% had minimal improvement, and 49% had no improvement. There is no evidence that Refissa treats coarse skin, deep wrinkles, yellowing skin, or other skin care problems.
Refissa should be used as part of a broad skin care program. This program should include avoiding direct sunlight (by using protective clothing and sunscreens with a minimum SPF of 15) and using other moisturizing facial creams that do not contain tretinoin. Many people can achieve desired effects by using this program without using Refissa. You should not use Refissa until you have tried a broad skin treatment program without Refissa.
When you use Refissa, improvement in fine wrinkling, spotty skin discoloration and rough skin is not immediate and occurs gradually over time. Generally, you may notice some effects in 3 to 4 months. The effects are usually most noticeable at about 6 months with little additional improvement after that time. If Refissa is stopped, the improvement will gradually diminish.
The safety of using Refissa daily for more than 48 weeks has not been established.
Who Should Not Use Refissa?
You should not use Refissa if you are sunburned or highly sensitive to the sun, if you have eczema, or if your skin is irritated. Refissa can cause increased skin irritation and increased susceptibility to sunburn.
Since Refissa may make your skin more sensitive to sunlight, you should tell your health care professional if you are also using other medicines that increase sensitivity to sunlight because you should not be using Refissa with these medicines. These include but are not limited to: thiazides (used to treat high blood pressure), tetracyclines, fluoroquinolones or sulfonamides (used to treat infection), and phenothiazines (used to treat serious emotional problems). If you are taking any prescription medicines, non-prescription medicines or using any facial creams, check with a health care professional to make sure they do not interact with Refissa.
Pregnancy Warning: Safe use during pregnancy has not been shown. There are reports of birth defects with laboratory animals and humans that were given tretinoin by mouth. You should not use Refissa [Tretinoin Cream, USP (Emollient) 0.05%] if you are pregnant or trying to become pregnant.
It is not known if Refissa is passed to infants through breast milk. Safe use in children has not been shown.
The safety and effectiveness of Refissa for people over age 50 or with darker skin coloration has not been proven.
How should I use Refissa?
You should apply Refissa to your face once a day before retiring using only enough to cover the entire affected area lightly. Gently wash your face with a mild soap, pat the skin dry, and wait 20 to 30 minutes before applying Refissa. Apply a pea-sized amount of cream to cover your entire face. You may feel a warmth or slight stinging when Refissa is first applied.
You must be especially careful when applying the cream to avoid your eyes, ears, nostrils, or mouth. Refissa may cause severe redness, itching, burning, stinging, and peeling if applied to these areas.
Using larger than necessary amounts of Refissa will not speed results and can cause an overdose. An overdose can result in red and peeling skin as well as some pain or discomfort.
You may use cosmetics after applying Refissa. Make sure to clean your face thoroughly before applying Refissa again.
What should I avoid while using Refissa?
Refissa increases your sensitivity to sunlight. Avoid sunlight as much as possible. Use protective clothing and a sunscreen with a minimum SPF of 15. Do not sunbathe or use sunlamps. If you are sensitive to sunlight or have a job that requires you to be out in the sun for long periods, you must use extreme caution.
While using Refissa, avoid any products that can dry or irritate the skin. For example, avoid products applied to the skin that contain alcohol, spices, or lime. Also, avoid cleansers, hair removal, or other products that can irritate the skin.
What Are the Possible Side Effects of Refissa?
The most common side effects are skin reactions. Itching, red, and dry skin have been reported. So have burning, stinging, and peeling skin. These are most often mild and are most common when treatment is started.
How Can I Get Additional Information?
This leaflet summarizes the most important information about Refissa. If you would like more information, talk to your doctor or other health care provider. There is also a leaflet written for health professionals that your pharmacist can provide for you.
CALL YOUR DOCTOR FOR MEDICAL ADVICE ABOUT SIDE EFFECTS. YOU ARE ENCOURAGED TO REPORT NEGATIVE SIDE EFFECTS OF PRESCRIPTION DRUGS TO THE FDA. VISIT WWW.FDA.GOV/MEDWATCH, OR CALL 1-800-FDA-1088.
Suneva Medical Inc.
San Diego, CA 92121
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - 40g carton label
NDC 50224-002-03
Refissa
Tretinoin Cream, USP(Emollient) 0.05 %
Rx Only NET WT 40 g
Tretinoin-40g

PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - 20g carton label
NDC 50224-002-06
Refissa
Tretinoin Cream, USP(Emollient) 0.05 %
Rx Only NET WT 20 g
Tretinoin-20g

PACKAGE LABEL. PRINCIPAL DISPLAY PANEL - Obagi Medical 20g carton label
NDC 50224-002-02
Refissa
Tretinoin Cream, USP(Emollient) 0.05 %
Rx Only NET WT 20 g
Obagi-Medical-20g

REFISSATRETINOIN CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||