RELENZA
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RELENZA safely and effectively. See full prescribing information for RELENZA. RELENZA (zanamivir) Inhalation Powder, for oral inhalationInitial U.S. Approval: 1999RECENT MAJOR CHANGESWarnings and Precautions (5.6) 12/2010INDICATIONS AND USAGERELENZA, an influenza neuraminidase inhibitor, is indicated for: Treatment of influenza in patients 7 years of age and older who have been symptomatic for no more than 2 days. (1.1) Prophylaxis of influenza in patients 5 years of age and older. (1.2) Important Limitations on Use of RELENZA: Not recommended for treatment or prophylaxis of influenza in: Individuals with underlying airways disease. (5.1) Not proven effective for: Treatment in individuals with underlying airways disease. (1.3) Prophylaxis in nursing home residents. (1.3) Not a substitute for annual influenza vaccination. (1.3) Consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use RELENZA. (1.3) DOSAGE AND ADMINISTRATION Indication Dose Treatment of Influenza (2.2) 10 mg twice daily for 5 days Prophylaxis: (2.3) Household Setting 10 mg once daily for 10 days Community Outbreaks 10 mg once daily for 28 days Note: The 10 mg dose is provided by 2 inhalations (one 5 mg blister per inhalation). (2.1)DOSAGE FORMS AND STRENGTHSBlister for oral inhalation: 5 mg. Four 5 mg blisters of powder on a ROTADISK® for oral inhalation via DISKHALER®. Packaged in carton containing 5 ROTADISKs (total of 10 doses) and 1 DISKHALER inhalation device. (3)CONTRAINDICATIONSDo not use in patients with history of allergic reaction to any ingredient of RELENZA, including lactose (which contains milk proteins). (4)WARNINGS AND PRECAUTIONS Bronchospasm: Serious, sometimes fatal, cases have occurred. Not recommended in individuals with underlying airways disease. Discontinue RELENZA if bronchospasm or decline in respiratory function develops. (5.1) Allergic Reactions: Discontinue RELENZA and initiate appropriate treatment if an allergic reaction occurs or is suspected. (5.2) Neuropsychiatric Events: Patients with influenza, particularly pediatric patients, may be at an increased risk of seizures, confusion, or abnormal behavior early in their illness. Monitor for signs of abnormal behavior. (5.3) High-risk Underlying Medical Conditions: Safety and effectiveness have not been demonstrated in these patients. (5.4) Side EffectsThe most common adverse events reported in >1.5% of patients treated with RELENZA and more commonly than in patients treated with placebo are: Treatment Studies – sinusitis, dizziness. Prophylaxis Studies – fever and/or chills, arthralgia and articular rheumatism. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSLive attenuated influenza vaccine, intranasal (7): Do not administer until 48 hours following cessation of RELENZA. Do not administer RELENZA until 2 weeks following administration of the live attenuated influenza vaccine, unless medically indicated. Revised: September 2009RLZ:6PI
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 RELENZA INDICATIONS AND USAGE
- 2 RELENZA DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 RELENZA CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 RELENZA ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 RELENZA DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Treatment of Influenza
RELENZA® (zanamivir) Inhalation Powder is indicated for treatment of uncomplicated acute illness due to influenza A and B virus in adults and pediatric patients 7 years of age and older who have been symptomatic for no more than 2 days.
1.2 Prophylaxis of Influenza
RELENZA is indicated for prophylaxis of influenza in adults and pediatric patients 5 years of age and older.
1.3 Important Limitations on Use of RELENZA
-
RELENZA is not recommended for treatment or prophylaxis of influenza in individuals with underlying airways disease (such as asthma or chronic obstructive pulmonary disease) due to risk of serious bronchospasm [see Warnings and Precautions (5.1)].
- RELENZA has not been proven effective for treatment of influenza in individuals with underlying airways disease.
- RELENZA has not been proven effective for prophylaxis of influenza in the nursing home setting.
- RELENZA is not a substitute for early influenza vaccination on an annual basis as recommended by the Centers for Disease Control's Immunization Practices Advisory Committee.
- Influenza viruses change over time. Emergence of resistance mutations could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use RELENZA.
- There is no evidence for efficacy of zanamivir in any illness caused by agents other than influenza virus A and B.
- Patients should be advised that the use of RELENZA for treatment of influenza has not been shown to reduce the risk of transmission of influenza to others.
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Considerations
-
RELENZA is for administration to the respiratory tract by oral inhalation only, using the DISKHALER device provided.
- The 10 mg dose is provided by 2 inhalations (one 5 mg blister per inhalation).
- Patients should be instructed in the use of the delivery system. Instructions should include a demonstration whenever possible. If RELENZA is prescribed for children, it should be used only under adult supervision and instruction, and the supervising adult should first be instructed by a healthcare professional [see Patient Counseling Information (17.4)].
- Patients scheduled to use an inhaled bronchodilator at the same time as RELENZA should use their bronchodilator before taking RELENZA [see Patient Counseling Information (17.2)].
2.2 Treatment of Influenza
-
The recommended dose of RELENZA for treatment of influenza in adults and pediatric patients 7 years of age and older is 10 mg twice daily (approximately 12 hours apart) for 5 days.
- Two doses should be taken on the first day of treatment whenever possible provided there is at least 2 hours between doses.
- On subsequent days, doses should be about 12 hours apart (e.g., morning and evening) at approximately the same time each day.
- The safety and efficacy of repeated treatment courses have not been studied.
2.3 Prophylaxis of Influenza
Household Setting:
- The recommended dose of RELENZA for prophylaxis of influenza in adults and pediatric patients 5 years of age and older in a household setting is 10 mg once daily for 10 days.
- The dose should be administered at approximately the same time each day.
- There are no data on the effectiveness of prophylaxis with RELENZA in a household setting when initiated more than 1.5 days after the onset of signs or symptoms in the index case.
Community Outbreaks:
- The recommended dose of RELENZA for prophylaxis of influenza in adults and adolescents in a community setting is 10 mg once daily for 28 days.
- The dose should be administered at approximately the same time each day.
- There are no data on the effectiveness of prophylaxis with RELENZA in a community outbreak when initiated more than 5 days after the outbreak was identified in the community.
- The safety and effectiveness of prophylaxis with RELENZA have not been evaluated for longer than 28 days’ duration.
3 DOSAGE FORMS AND STRENGTHS
Blister for oral inhalation: 5 mg. Four 5 mg blisters of powder on a ROTADISK for oral inhalation via DISKHALER. Packaged in carton containing 5 ROTADISKs (total of 10 doses) and 1 DISKHALER inhalation device [see How Supplied/Storage and Handling (16)].
4 CONTRAINDICATIONS
Do not use in patients with history of allergic reaction to any ingredient of RELENZA including lactose (which contains milk proteins) [see Warnings and Precautions (5.2), Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
RELENZA is not recommended for treatment or prophylaxis of influenza in individuals with underlying airways disease (such as asthma or chronic obstructive pulmonary disease).
Serious cases of bronchospasm, including fatalities, have been reported during treatment with RELENZA in patients with and without underlying airways disease. Many of these cases were reported during postmarketing and causality was difficult to assess.
RELENZA should be discontinued in any patient who develops bronchospasm or decline in respiratory function; immediate treatment and hospitalization may be required.
Some patients without prior pulmonary disease may also have respiratory abnormalities from acute respiratory infection that could resemble adverse drug reactions or increase patient vulnerability to adverse drug reactions.
Bronchospasm was documented following administration of zanamivir in 1 of 13 patients with mild or moderate asthma (but without acute influenza-like illness) in a Phase I study. In a Phase III study in patients with acute influenza-like illness superimposed on underlying asthma or chronic obstructive pulmonary disease, 10% (24 of 244) of patients on zanamivir and 9% (22 of 237) on placebo experienced a greater than 20% decline in FEV1 following treatment for 5 days.
If use of RELENZA is considered for a patient with underlying airways disease, the potential risks and benefits should be carefully weighed. If a decision is made to prescribe RELENZA for such a patient, this should be done only under conditions of careful monitoring of respiratory function, close observation, and appropriate supportive care including availability of fast-acting bronchodilators.
5.2 Allergic Reactions
Allergic-like reactions, including oropharyngeal edema, serious skin rashes, and anaphylaxis have been reported in postmarketing experience with RELENZA. RELENZA should be stopped and appropriate treatment instituted if an allergic reaction occurs or is suspected.
5.3 Neuropsychiatric Events
Influenza can be associated with a variety of neurologic and behavioral symptoms which can include events such as seizures, hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease.
There have been postmarketing reports (mostly from Japan) of delirium and abnormal behavior leading to injury in patients with influenza who were receiving neuraminidase inhibitors, including RELENZA. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made, but they appear to be uncommon based on usage data for RELENZA. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of RELENZA to these events has not been established. Patients with influenza should be closely monitored for signs of abnormal behavior. If neuropsychiatric symptoms occur, the risks and benefits of continuing treatment should be evaluated for each patient.
5.4 Limitations of Populations Studied
Safety and efficacy have not been demonstrated in patients with high-risk underlying medical conditions. No information is available regarding treatment of influenza in patients with any medical condition sufficiently severe or unstable to be considered at imminent risk of requiring inpatient management.
5.5 Bacterial Infections
Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. RELENZA has not been shown to prevent such complications.
RELENZA Inhalation Powder must not be made into an extemporaneous solution for administration by nebulization or mechanical ventilation. There have been reports of hospitalized patients with influenza who received a solution made with RELENZA Inhalation Powder administered by nebulization or mechanical ventilation, including a fatal case where it was reported that the lactose in this formulation obstructed the proper functioning of the equipment. RELENZA Inhalation Powder must only be administered using the device provided [see Dosage and Administration (2.1)].
5.7 Importance of Proper Use of DISKHALER
Effective and safe use of RELENZA requires proper use of the DISKHALER to inhale the drug. Prescribers should carefully evaluate the ability of young children to use the delivery system if use of RELENZA is considered [see Use in Specific Populations (8.4)].
6 ADVERSE REACTIONS
See Warnings and Precautions for information about risk of serious adverse events such as bronchospasm (5.1) and allergic-like reactions (5.2), and for safety information in patients with underlying airways disease (5.1).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The placebo used in clinical studies consisted of inhaled lactose powder, which is also the vehicle for the active drug; therefore, some adverse events occurring at similar frequencies in different treatment groups could be related to lactose vehicle inhalation.
Treatment of Influenza: Clinical Trials in Adults and Adolescents: Adverse events that occurred with an incidence ≥1.5% in treatment studies are listed in Table 1. This table shows adverse events occurring in patients ≥12 years of age receiving RELENZA 10 mg inhaled twice daily, RELENZA in all inhalation regimens, and placebo inhaled twice daily (where placebo consisted of the same lactose vehicle used in RELENZA).
| Adverse Event | RELENZA |
Placebo (Lactose Vehicle) (n = 1,520) |
|
|
10 mg b.i.d. Inhaled (n = 1,132) |
All Dosing Regimensa (n = 2,289) |
||
| Body as a whole | |||
| Headaches | 2% | 2% | 3% |
| Digestive | |||
| Diarrhea | 3% | 3% | 4% |
| Nausea | 3% | 3% | 3% |
| Vomiting | 1% | 1% | 2% |
| Respiratory | |||
| Nasal signs and symptoms | 2% | 3% | 3% |
| Bronchitis | 2% | 2% | 3% |
| Cough | 2% | 2% | 3% |
| Sinusitis | 3% | 2% | 2% |
| Ear, nose, and throat infections | 2% | 1% | 2% |
| Nervous system | |||
| Dizziness | 2% | 1% | <1% |
| a Includes studies where RELENZA was administered intranasally (6.4 mg 2 to 4 times per day in addition to inhaled preparation) and/or inhaled more frequently (q.i.d.) than the currently recommended dose. | |||
Additional adverse reactions occurring in less than 1.5% of patients receiving RELENZA included malaise, fatigue, fever, abdominal pain, myalgia, arthralgia, and urticaria.
The most frequent laboratory abnormalities in Phase III treatment studies included elevations of liver enzymes and CPK, lymphopenia, and neutropenia. These were reported in similar proportions of zanamivir and lactose vehicle placebo recipients with acute influenza-like illness.
Clinical Trials in Pediatric Patients: Adverse events that occurred with an incidence ≥1.5% in children receiving treatment doses of RELENZA in 2 Phase III studies are listed in Table 2. This table shows adverse events occurring in pediatric patients 5 to 12 years old receiving RELENZA 10 mg inhaled twice daily and placebo inhaled twice daily (where placebo consisted of the same lactose vehicle used in RELENZA).
| a Includes a subset of patients receiving RELENZA for treatment of influenza in a prophylaxis study. | ||
| Adverse Event |
RELENZA 10 mg b.i.d. Inhaled (n = 291) |
Placebo (Lactose Vehicle) (n = 318) |
| Respiratory | ||
| Ear, nose, and throat infections | 5% | 5% |
| Ear, nose, and throat hemorrhage | <1% | 2% |
| Asthma | <1% | 2% |
| Cough | <1% | 2% |
| Digestive | ||
| Vomiting | 2% | 3% |
| Diarrhea | 2% | 2% |
| Nausea | <1% | 2% |
In 1 of the 2 studies described in Table 2, some additional information is available from children (5 to 12 years old) without acute influenza-like illness who received an investigational prophylaxis regimen of RELENZA; 132 children received RELENZA and 145 children received placebo. Among these children, nasal signs and symptoms (zanamivir 20%, placebo 9%), cough (zanamivir 16%, placebo 8%), and throat/tonsil discomfort and pain (zanamivir 11%, placebo 6%) were reported more frequently with RELENZA than placebo. In a subset with chronic pulmonary disease, lower respiratory adverse events (described as asthma, cough, or viral respiratory infections which could include influenza-like symptoms) were reported in 7 of 7 zanamivir recipients and 5 of 12 placebo recipients.
Prophylaxis of Influenza: Family/Household Prophylaxis Studies: Adverse events that occurred with an incidence of ≥1.5% in the 2 prophylaxis studies are listed in Table 3. This table shows adverse events occurring in patients ≥5 years of age receiving RELENZA 10 mg inhaled once daily for 10 days.
| a In prophylaxis studies, symptoms associated with influenza-like illness were captured as adverse events; subjects were enrolled during a winter respiratory season during which time any symptoms that occurred were captured as adverse events. | ||
| Adverse Event | Contact Cases | |
|
RELENZA (n = 1,068) |
Placebo (n = 1,059) |
|
| Lower respiratory | ||
| Viral respiratory infections | 13% | 19% |
| Cough | 7% | 9% |
| Neurologic | ||
| Headaches | 13% | 14% |
| Ear, nose, and throat | ||
| Nasal signs and symptoms | 12% | 12% |
| Throat and tonsil discomfort and pain | 8% | 9% |
| Nasal inflammation | 1% | 2% |
| Musculoskeletal | ||
| Muscle pain | 3% | 3% |
| Endocrine and metabolic | ||
| Feeding problems (decreased or increased appetite and anorexia) | 2% | 2% |
| Gastrointestinal | ||
| Nausea and vomiting | 1% | 2% |
| Non-site specific | ||
| Malaise and fatigue | 5% | 5% |
| Temperature regulation disturbances (fever and/or chills) | 5% | 4% |
Community Prophylaxis Studies: Adverse events that occurred with an incidence of ≥1.5% in 2 prophylaxis studies are listed in Table 4. This table shows adverse events occurring in patients ≥5 years of age receiving RELENZA 10 mg inhaled once daily for 28 days.
| a In prophylaxis studies, symptoms associated with influenza-like illness were captured as adverse events; subjects were enrolled during a winter respiratory season during which time any symptoms that occurred were captured as adverse events. | ||
| Adverse Event |
RELENZA (n = 2,231) |
Placebo (n = 2,239) |
| Neurologic | ||
| Headaches | 24% | 26% |
| Ear, nose, and throat | ||
| Throat and tonsil discomfort and pain | 19% | 20% |
| Nasal signs and symptoms | 12% | 13% |
| Ear, nose, and throat infections | 2% | 2% |
| Lower respiratory | ||
| Cough | 17% | 18% |
| Viral respiratory infections | 3% | 4% |
| Musculoskeletal | ||
| Muscle pain | 8% | 8% |
| Musculoskeletal pain | 6% | 6% |
| Arthralgia and articular rheumatism | 2% | <1% |
| Endocrine and metabolic | ||
| Feeding problems (decreased or increased appetite and anorexia) | 4% | 4% |
| Gastrointestinal | ||
| Nausea and vomiting | 2% | 3% |
| Diarrhea | 2% | 2% |
| Non-site specific | ||
| Temperature regulation disturbances (fever and/or chills) | 9% | 10% |
| Malaise and fatigue | 8% | 8% |
6.2 Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postmarketing use of zanamivir (RELENZA). Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to zanamivir (RELENZA).
Allergic Reactions: Allergic or allergic-like reaction, including oropharyngeal edema [see Warnings and Precautions (5.2)].
Psychiatric: Delirium, including symptoms such as altered level of consciousness, confusion, abnormal behavior, delusions, hallucinations, agitation, anxiety, nightmares[see Warnings and Precautions (5.3)].
Cardiac: Arrhythmias, syncope.
Neurologic: Seizures.
Respiratory: Bronchospasm, dyspnea [see Warnings and Precautions (5.1)].
Skin: Facial edema; rash, including serious cutaneous reactions (e.g., erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis); urticaria [see Warnings and Precautions (5.2)].
7 DRUG INTERACTIONS
Zanamivir is not a substrate nor does it affect cytochrome P450 (CYP) isoenzymes (CYP1A1/2, 2A6, 2C9, 2C18, 2D6, 2E1, and 3A4) in human liver microsomes. No clinically significant pharmacokinetic drug interactions are predicted based on data from in vitro studies.
The concurrent use of RELENZA with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of potential interference between these products, LAIV should not be administered within 2 weeks before or 48 hours after administration of RELENZA, unless medically indicated. The concern about possible interference arises from the potential for antiviral drugs to inhibit replication of live vaccine virus.
Trivalent inactivated influenza vaccine can be administered at any time relative to use of RELENZA [see Clinical Pharmacology (12.4)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. There are no adequate and well-controlled studies of zanamivir in pregnant women. Zanamivir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Embryo/fetal development studies were conducted in rats (dosed from days 6 to 15 of pregnancy) and rabbits (dosed from days 7 to 19 of pregnancy) using the same IV doses (1, 9, and 90 mg/kg/day). Pre- and post-natal developmental studies were performed in rats (dosed from day 16 of pregnancy until litter day 21 to 23). No malformations, maternal toxicity, or embryotoxicity were observed in pregnant rats or rabbits and their fetuses. Because of insufficient blood sampling timepoints in rat and rabbit reproductive toxicity studies, AUC values were not available. In a subchronic study in rats at the 90 mg/kg/day IV dose, the AUC values were greater than 300 times the human exposure at the proposed clinical dose.
An additional embryo/fetal study, in a different strain of rat, was conducted using subcutaneous administration of zanamivir, 3 times daily, at doses of 1, 9, or 80 mg/kg during days 7 to 17 of pregnancy. There was an increase in the incidence rates of a variety of minor skeleton alterations and variants in the exposed offspring in this study. Based on AUC measurements, the 80 mg/kg dose produced an exposure greater than 1,000 times the human exposure at the proposed clinical dose. However, in most instances, the individual incidence rate of each skeletal alteration or variant remained within the background rates of the historical occurrence in the strain studied.
Zanamivir has been shown to cross the placenta in rats and rabbits. In these animals, fetal blood concentrations of zanamivir were significantly lower than zanamivir concentrations in the maternal blood.
8.3 Nursing Mothers
Studies in rats have demonstrated that zanamivir is excreted in milk. However, nursing mothers should be instructed that it is not known whether zanamivir is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when RELENZA is administered to a nursing mother.
8.4 Pediatric Use
Treatment of Influenza: Safety and effectiveness of RELENZA for treatment of influenza have not been assessed in pediatric patients less than 7 years of age, but were studied in a Phase III treatment study in pediatric patients, where 471 children 5 to 12 years of age received zanamivir or placebo [see Clinical Studies 14.1)]. Adolescents were included in the 3 principal Phase III adult treatment studies. In these studies, 67 patients were 12 to 16 years of age. No definite differences in safety and efficacy were observed between these adolescent patients and young adults.
In a Phase I study of 16 children ages 6 to 12 years with signs and symptoms of respiratory disease, 4 did not produce a measurable peak inspiratory flow rate (PIFR) through the DISKHALER (3 with no adequate inhalation on request, 1 with missing data), 9 had measurable PIFR on each of 2 inhalations, and 3 achieved measurable PIFR on only 1 of 2 inhalations. Neither of two 6-year-olds and one of two 7-year-olds produced measurable PIFR. Overall, 8 of the 16 children (including all those under 8 years old) either did not produce measurable inspiratory flow through the DISKHALER or produced peak inspiratory flow rates below the 60 L/min considered optimal for the device under standardized in vitro testing; lack of measurable flow rate was related to low or undetectable serum concentrations [see Clinical Pharmacology (12.3), Clinical Studies (14.1)]. Prescribers should carefully evaluate the ability of young children to use the delivery system if prescription of RELENZA is considered.
Prophylaxis of Influenza: The safety and effectiveness of RELENZA for prophylaxis of influenza have been studied in 4 Phase III studies where 273 children 5 to 11 years of age and 239 adolescents 12 to 16 years of age received RELENZA. No differences in safety and effectiveness were observed between pediatric and adult subjects [see Clinical Studies (14.2)].
8.5 Geriatric Use
Of the total number of patients in 6 clinical studies of RELENZA for treatment of influenza, 59 patients were 65 years of age and older, while 24 patients were 75 years of age and older. Of the total number of patients in 4 clinical studies of RELENZA for prophylaxis of influenza in households and community settings, 954 patients were 65 years of age and older, while 347 patients were 75 years of age and older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Elderly patients may need assistance with use of the device.
In 2 additional studies of RELENZA for prophylaxis of influenza in the nursing home setting, efficacy was not demonstrated [see Indications and Usage (1.3)].
10 OVERDOSAGE
There have been no reports of overdosage from administration of RELENZA.
11 DESCRIPTION
The active component of RELENZA is zanamivir. The chemical name of zanamivir is 5-(acetylamino)-4-[(aminoiminomethyl)-amino]-2,6-anhydro-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonic acid. It has a molecular formula of C12H20N4O7 and a molecular weight of 332.3. It has the following structural formula:
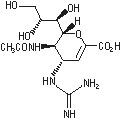
Zanamivir is a white to off-white powder for oral inhalation with a solubility of approximately 18 mg/mL in water at 20°C.
RELENZA is for administration to the respiratory tract by oral inhalation only. Each RELENZA ROTADISK contains 4 regularly spaced double-foil blisters with each blister containing a powder mixture of 5 mg of zanamivir and 20 mg of lactose (which contains milk proteins). The contents of each blister are inhaled using a specially designed breath-activated plastic device for inhaling powder called the DISKHALER. After a RELENZA ROTADISK is loaded into the DISKHALER, a blister that contains medication is pierced and the zanamivir is dispersed into the air stream created when the patient inhales through the mouthpiece. The amount of drug delivered to the respiratory tract will depend on patient factors such as inspiratory flow. Under standardized in vitro testing, RELENZA ROTADISK delivers 4 mg of zanamivir from the DISKHALER device when tested at a pressure drop of 3 kPa (corresponding to a flow rate of about 62 to 65 L/min) for 3 seconds.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Zanamivir is an antiviral drug [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Absorption and Bioavailability: Pharmacokinetic studies of orally inhaled zanamivir indicate that approximately 4% to 17% of the inhaled dose is systemically absorbed. The peak serum concentrations ranged from 17 to 142 ng/mL within 1 to 2 hours following a 10 mg dose. The area under the serum concentration versus time curve (AUC∞) ranged from 111 to 1,364 ng•hr/mL.
Distribution: Zanamivir has limited plasma protein binding (<10%).
Metabolism: Zanamivir is renally excreted as unchanged drug. No metabolites have been detected in humans.
Elimination: The serum half-life of zanamivir following administration by oral inhalation ranges from 2.5 to 5.1 hours. It is excreted unchanged in the urine with excretion of a single dose completed within 24 hours. Total clearance ranges from 2.5 to 10.9 L/hr. Unabsorbed drug is excreted in the feces.
Impaired Hepatic Function: The pharmacokinetics of zanamivir have not been studied in patients with impaired hepatic function.
Impaired Renal Function: After a single intravenous dose of 4 mg or 2 mg of zanamivir in volunteers with mild/moderate or severe renal impairment, respectively, significant decreases in renal clearance (and hence total clearance: normals 5.3 L/hr, mild/moderate 2.7 L/hr, and severe 0.8 L/hr; median values) and significant increases in half-life (normals 3.1 hr, mild/moderate 4.7 hr, and severe 18.5 hr; median values) and systemic exposure were observed. Safety and efficacy have not been documented in the presence of severe renal insufficiency. Due to the low systemic bioavailability of zanamivir following oral inhalation, no dosage adjustments are necessary in patients with renal impairment. However, the potential for drug accumulation should be considered.
Pediatric Patients: The pharmacokinetics of zanamivir were evaluated in pediatric patients with signs and symptoms of respiratory illness. Sixteen patients, 6 to 12 years of age, received a single dose of 10 mg zanamivir dry powder via DISKHALER. Five patients had either undetectable zanamivir serum concentrations or had low drug concentrations (8.32 to 10.38 ng/mL) that were not detectable after 1.5 hours. Eleven patients had Cmax median values of 43 ng/mL (range 15 to 74) and AUC∞ median values of 167 ng•hr/mL (range 58 to 279). Low or undetectable serum concentrations were related to lack of measurable PIFR in individual patients [see Use in Specific Populations (8.4), Clinical Studies (14.1)].
Geriatric Patients: The pharmacokinetics of zanamivir have not been studied in patients over 65 years of age [see Use in Specific Populations (8.5)].
Gender, Race, and Weight: In a population pharmacokinetic analysis in patient studies, no clinically significant differences in serum concentrations and/or pharmacokinetic parameters (V/F, CL/F, ka, AUC0-3, Cmax, Tmax, CLr, and % excreted in urine) were observed when demographic variables (gender, age, race, and weight) and indices of infection (laboratory evidence of infection, overall symptoms, symptoms of upper respiratory illness, and viral titers) were considered. There were no significant correlations between measures of systemic exposure and safety parameters.
12.4 Microbiology
Mechanism of Action: Zanamivir is an inhibitor of influenza virus neuraminidase affecting release of viral particles.
Antiviral Activity: The antiviral activity of zanamivir against laboratory and clinical isolates of influenza virus was determined in cell culture assays. The concentrations of zanamivir required for inhibition of influenza virus were highly variable depending on the assay method used and virus isolate tested. The 50% and 90% effective concentrations (EC50 and EC90) of zanamivir were in the range of 0.005 to 16.0 μM and 0.05 to >100 μM, respectively (1 μM = 0.33 mcg/mL). The relationship between the cell culture inhibition of influenza virus by zanamivir and the inhibition of influenza virus replication in humans has not been established.
Resistance: Influenza viruses with reduced susceptibility to zanamivir have been selected in cell culture by multiple passages of the virus in the presence of increasing concentrations of the drug. Genetic analysis of these viruses showed that the reduced susceptibility in cell culture to zanamivir is associated with mutations that result in amino acid changes in the viral neuraminidase or viral hemagglutinin or both. Resistance mutations selected in cell culture which result in neuraminidase amino acid substitutions include E119G/A/D and R292K. Mutations selected in cell culture in hemagglutinin include: K68R, G75E, E114K, N145S, S165N, S186F, N199S, and K222T.
In an immunocompromised patient infected with influenza B virus, a variant virus emerged after treatment with an investigational nebulized solution of zanamivir for 2 weeks. Analysis of this variant showed a hemagglutinin substitution (T198I) which resulted in a reduced affinity for human cell receptors, and a substitution in the neuraminidase active site (R152K) which reduced the enzyme’s activity to zanamivir by 1,000-fold. Insufficient information is available to characterize the risk of emergence of zanamivir resistance in clinical use.
Cross-Resistance: Cross-resistance has been observed between some zanamivir-resistant and some oseltamivir-resistant influenza virus mutants generated in cell culture. However, some of the in cell culture zanamivir-induced resistance mutations, E119G/A/D and R292K, occurred at the same neuraminidase amino acid positions as in the clinical isolates resistant to oseltamivir, E119V and R292K. No studies have been performed to assess risk of emergence of cross-resistance during clinical use.
Influenza Vaccine Interaction Study: An interaction study (n = 138) was conducted to evaluate the effects of zanamivir (10 mg once daily) on the serological response to a single dose of trivalent inactivated influenza vaccine, as measured by hemagglutination inhibition titers. There was no difference in hemagglutination inhibition antibody titers at 2 weeks and 4 weeks after vaccine administration between zanamivir and placebo recipients.
Influenza Challenge Studies: Antiviral activity of zanamivir was supported for infection with influenza A virus, and to a more limited extent for infection with influenza B virus, by Phase I studies in volunteers who received intranasal inoculations of challenge strains of influenza virus, and received an intranasal formulation of zanamivir or placebo starting before or shortly after viral inoculation.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: In 2-year carcinogenicity studies conducted in rats and mice using a powder formulation administered through inhalation, zanamivir induced no statistically significant increases in tumors over controls. The maximum daily exposures in rats and mice were approximately 23 to 25 and 20 to 22 times, respectively, greater than those in humans at the proposed clinical dose based on AUC comparisons.
Mutagenesis: Zanamivir was not mutagenic in in vitro and in vivo genotoxicity assays which included bacterial mutation assays in S. typhimurium and E. coli, mammalian mutation assays in mouse lymphoma, chromosomal aberration assays in human peripheral blood lymphocytes, and the in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility: The effects of zanamivir on fertility and general reproductive performance were investigated in male (dosed for 10 weeks prior to mating, and throughout mating, gestation/lactation, and shortly after weaning) and female rats (dosed for 3 weeks prior to mating through Day 19 of pregnancy, or Day 21 post partum) at IV doses 1, 9, and 90 mg/kg/day. Zanamivir did not impair mating or fertility of male or female rats, and did not affect the sperm of treated male rats. The reproductive performance of the F1 generation born to female rats given zanamivir was not affected. Based on a subchronic study in rats at a 90 mg/kg/day IV dose, AUC values ranged between 142 and 199 mcg•hr/mL (>300 times the human exposure at the proposed clinical dose).
14 CLINICAL STUDIES
14.1 Treatment of Influenza
Adults and Adolescents: The efficacy of RELENZA 10 mg inhaled twice daily for 5 days in the treatment of influenza has been evaluated in placebo-controlled studies conducted in North America, the Southern Hemisphere, and Europe during their respective influenza seasons. The magnitude of treatment effect varied between studies, with possible relationships to population-related factors including amount of symptomatic relief medication used.
Populations Studied: The principal Phase III studies enrolled 1,588 patients ages 12 years and older (median age 34 years, 49% male, 91% Caucasian), with uncomplicated influenza-like illness within 2 days of symptom onset. Influenza was confirmed by culture, hemagglutination inhibition antibodies, or investigational direct tests. Of 1,164 patients with confirmed influenza, 89% had influenza A and 11% had influenza B. These studies served as the principal basis for efficacy evaluation, with more limited Phase II studies providing supporting information where necessary. Following randomization to either zanamivir or placebo (inhaled lactose vehicle), all patients received instruction and supervision by a healthcare professional for the initial dose.
Principal Results: The definition of time to improvement in major symptoms of influenza included no fever and self-assessment of “none” or “mild” for headache, myalgia, cough, and sore throat. A Phase II and a Phase III study conducted in North America (total of over 600 influenza-positive patients) suggested up to 1 day of shortening of median time to this defined improvement in symptoms in patients receiving zanamivir compared with placebo, although statistical significance was not reached in either of these studies. In a study conducted in the Southern Hemisphere (321 influenza-positive patients), a 1.5-day difference in median time to symptom improvement was observed. Additional evidence of efficacy was provided by the European study.
Other Findings: There was no consistent difference in treatment effect in patients with influenza A compared with influenza B; however, these trials enrolled smaller numbers of patients with influenza B and thus provided less evidence in support of efficacy in influenza B.
In general, patients with lower temperature (e.g., 38.2°C or less) or investigator-rated as having less severe symptoms at entry derived less benefit from therapy.
No consistent treatment effect was demonstrated in patients with underlying chronic medical conditions, including respiratory or cardiovascular disease [see Warnings and Precautions (5.4)].
No consistent differences in rate of development of complications were observed between treatment groups.
Some fluctuation of symptoms was observed after the primary study endpoint in both treatment groups.
Pediatric Patients: The efficacy of RELENZA 10 mg inhaled twice daily for 5 days in the treatment of influenza in pediatric patients has been evaluated in a placebo-controlled study conducted in North America and Europe, enrolling 471 patients, ages 5 to 12 years (55% male, 90% Caucasian), within 36 hours of symptom onset. Of 346 patients with confirmed influenza, 65% had influenza A and 35% had influenza B. The definition of time to improvement included no fever and parental assessment of no or mild cough and absent/minimal muscle and joint aches or pains, sore throat, chills/feverishness, and headache. Median time to symptom improvement was 1 day shorter in patients receiving zanamivir compared with placebo. No consistent differences in rate of development of complications were observed between treatment groups. Some fluctuation of symptoms was observed after the primary study endpoint in both treatment groups.
Although this study was designed to enroll children ages 5 to 12 years, the product is indicated only for children 7 years of age and older. This evaluation is based on the combination of lower estimates of treatment effect in 5- and 6-year-olds compared with the overall study population, and evidence of inadequate inhalation through the DISKHALER in a pharmacokinetic study [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3)].
14.2 Prophylaxis of Influenza
The efficacy of RELENZA in preventing naturally occurring influenza illness has been demonstrated in 2 post-exposure prophylaxis studies in households and 2 seasonal prophylaxis studies during community outbreaks of influenza. The primary efficacy endpoint in these studies was the incidence of symptomatic, laboratory-confirmed influenza, defined as the presence of 2 or more of the following symptoms: oral temperature ≥100°F/37.8°C or feverishness, cough, headache, sore throat, and myalgia; and laboratory confirmation of influenza A or B by culture, PCR, or seroconversion (defined as a 4-fold increase in convalescent antibody titer from baseline).
Household Prophylaxis Studies: Two studies assessed post-exposure prophylaxis in household contacts of an index case. Within 1.5 days of onset of symptoms in an index case, each household (including all family members ≥5 years of age) was randomized to RELENZA 10 mg inhaled once daily or placebo inhaled once daily for 10 days. In the first study only, each index case was randomized to RELENZA 10 mg inhaled twice daily for 5 days or inhaled placebo twice daily for 5 days. In this study, the proportion of households with at least 1 new case of symptomatic laboratory-confirmed influenza was reduced from 19.0% (32 of 168 households) for the placebo group to 4.1% (7 of 169 households) for the group receiving RELENZA.
In the second study, index cases were not treated. The incidence of symptomatic laboratory-confirmed influenza was reduced from 19.0% (46 of 242 households) for the placebo group to 4.1% (10 of 245 households) for the group receiving RELENZA.
Seasonal Prophylaxis Studies: Two seasonal prophylaxis studies assessed RELENZA 10 mg inhaled once daily versus placebo inhaled once daily for 28 days during community outbreaks. The first study enrolled subjects 18 years of age or greater (mean age 29 years) from 2 university communities. The majority of subjects were unvaccinated (86%). In this study, the incidence of symptomatic laboratory-confirmed influenza was reduced from 6.1% (34 of 554) for the placebo group to 2.0% (11 of 553) for the group receiving RELENZA.
The second seasonal prophylaxis study enrolled subjects 12 to 94 years of age (mean age 60 years) with 56% of them older than 65 years of age. Sixty-seven percent of the subjects were vaccinated. In this study, the incidence of symptomatic laboratory-confirmed influenza was reduced from 1.4% (23 of 1,685) for the placebo group to 0.2% (4 of 1,678) for the group receiving RELENZA.
16 HOW SUPPLIED/STORAGE AND HANDLING
RELENZA is supplied in a circular double-foil pack (a ROTADISK) containing 4 blisters of the drug. Five ROTADISKs are packaged in a white polypropylene tube. The tube is packaged in a carton with 1 blue and gray DISKHALER inhalation device.
They are supplied by Dispensing Solutions Inc. as follows:
| NDC | Strength | Quantity/Form | Source NDC |
| 54868-4377-0 | 5 mg | 5 Rotadisks containing 4 Blisters | 0173-0681-01 |
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). Keep out of reach of children. Do not puncture any RELENZA ROTADISK blister until taking a dose using the DISKHALER.
Manufactured By:
GlaxoSmithKline
Research Triangle Park, NC 27709
©2010, GlaxoSmithKline. All rights reserved.
Decembe 2010
RLZ:8PI
Relabeling of "Additional" label by:
Physicians Total Care, Inc.
Tulsa, OK 74146
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling provided as a separate leaflet accompanying the product.
17.1 Bronchospasm
Patients should be advised of the risk of bronchospasm, especially in the setting of underlying airways disease, and should stop RELENZA and contact their physician if they experience increased respiratory symptoms during treatment such as worsening wheezing, shortness of breath, or other signs or symptoms of bronchospasm [see Warnings and Precautions (5.1)] . If a decision is made to prescribe RELENZA for a patient with asthma or chronic obstructive pulmonary disease, the patient should be made aware of the risks and should have a fast-acting bronchodilator available.
17.2 Concomitant Bronchodilator Use
Patients scheduled to take inhaled bronchodilators at the same time as RELENZA should be advised to use their bronchodilators before taking RELENZA.
17.3 Neuropsychiatric Events
Patients with influenza (the flu), particularly children and adolescents, may be at an increased risk of seizures, confusion, or abnormal behavior early in their illness. These events may occur after beginning RELENZA or may occur when flu is not treated. These events are uncommon but may result in accidental injury to the patient. Therefore, patients should be observed for signs of unusual behavior and a healthcare professional should be contacted immediately if the patient shows any signs of unusual behavior [see Warnings and Precautions (5.3)].
17. 4 Instructions for Use
Patients should be instructed in use of the delivery system. Instructions should include a demonstration whenever possible. For the proper use of RELENZA, the patient should read and follow carefully the accompanying Patient Instructions for Use.
If RELENZA is prescribed for children, it should be used only under adult supervision and instruction, and the supervising adult should first be instructed by a healthcare professional [see Dosage and Administration (2.1)].
17.5 Risk of Influenza Transmission to Others
Patients should be advised that the use of RELENZA for treatment of influenza has not been shown to reduce the risk of transmission of influenza to others.
RELENZA, DISKHALER, and ROTADISK are registered trademarks of GlaxoSmithKline.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2009, GlaxoSmithKline. All rights reserved.
PATIENT LABELING
RELENZA® (zanamivir) Inhalation Powder
This leaflet contains important patient information about RELENZA (zanamivir) Inhalation Powder, and should be read completely before beginning treatment. It does not, however, take the place of discussions with your healthcare provider about your medical condition or your treatment. This summary does not list all benefits and risks of RELENZA. The medication described here can only be prescribed and dispensed by a licensed healthcare provider, who has information about your medical condition and more information about the drug, including how to take it, what to expect, and potential side effects. If you have any questions about RELENZA, talk with your healthcare provider.
What is RELENZA?
RELENZA (ruh-LENS-uh) is a medicine for the treatment of influenza (flu, infection caused by influenza virus) and for reducing the chance of getting the flu in community and household settings. It belongs to a group of medicines called neuraminidase inhibitors. These medications attack the influenza virus and prevent it from spreading inside your body. RELENZA treats the cause of influenza at its source, rather than simply masking the symptoms.
Important Safety Information About RELENZA
Some patients have had bronchospasm (wheezing) or serious breathing problems when they used RELENZA. Many but not all of these patients had previous asthma or chronic obstructive pulmonary disease. RELENZA has not been shown to shorten the duration of influenza in people with these diseases. Because of the risk of side effects and because it has not been shown to help them, RELENZA is not recommended for people with chronic respiratory disease such as asthma or chronic obstructive pulmonary disease.
If you develop worsening respiratory symptoms such as wheezing or shortness of breath, stop using RELENZA and contact your healthcare provider right away.
If you have chronic respiratory disease such as asthma and chronic obstructive pulmonary disease and your healthcare provider has prescribed RELENZA, you should have a fast-acting, inhaled bronchodilator available for your use. If you are scheduled to use an inhaled bronchodilator at the same time as RELENZA, use the inhaled bronchodilator before using RELENZA.
Read the rest of this leaflet for more information about side effects and risks.
Other kinds of infections can appear like influenza or occur along with influenza, and need different kinds of treatment. Contact your healthcare provider if you feel worse or develop new symptoms during or after treatment, or if your influenza symptoms do not start to get better.
Who should not take RELENZA?
RELENZA is not recommended for people who have chronic lung disease such as asthma or chronic obstructive pulmonary disease. RELENZA has not been shown to shorten the duration of influenza in people with these diseases, and some people have had serious side effects of bronchospasm and worsening lung function. (See the section of this Patient Information entitled “Important Safety Information About RELENZA.”)
You should not take RELENZA if you are allergic to zanamivir or any other ingredient of RELENZA. Also tell your healthcare provider if you have any type of chronic condition including lung or heart disease, if you are allergic to any other medicines or food products, or if you are pregnant.
RELENZA was not effective in reducing the chance of getting the flu in 2 studies in nursing home patients.
RELENZA does not treat flu-like illness that is not caused by influenza virus.
Who should consider taking RELENZA?
Adult and pediatric patients at least 7 years of age who have influenza symptoms that appeared within the previous day or two. Typical symptoms of influenza include sudden onset of fever, cough, headache, fatigue, muscular weakness, and sore throat.
RELENZA can also help reduce the chance of getting the flu in adults and children at least 5 years of age who have a higher chance of getting the flu because they spend time with someone who has the flu. RELENZA can also reduce the chance of getting the flu if there is a flu outbreak in the community.
The use of RELENZA for the treatment of flu has not been shown to reduce the risk of spreading the virus to others.
Can I take other medications with RELENZA?
RELENZA has been shown to have an acceptable safety profile when used as labeled, with minimal risk of drug interactions. Your healthcare provider may recommend taking other medications, including over-the-counter medications, to reduce fever or other symptoms while you are taking RELENZA. Before starting treatment, make sure that your healthcare provider knows if you are taking other medicines. If you are scheduled to use an inhaled bronchodilator at the same time as RELENZA, you should use the inhaled bronchodilator before using RELENZA.
Before taking RELENZA, please let your healthcare provider know if you received live attenuated influenza vaccine (FLUMIST®) intranasal in the past 2 weeks.
How and when should I take RELENZA?
RELENZA is packaged in medicine disks called ROTADISKS® and is inhaled by mouth using a delivery device called a DISKHALER®. Each ROTADISK contains 4 blisters. Each blister contains 5 mg of active drug and 20 mg of lactose powder (which contains milk proteins).
You should receive a demonstration on how to use RELENZA in the DISKHALER from a healthcare provider. Before taking RELENZA, read the “Patient Instructions for Use.” Make sure that you understand these instructions and talk to your healthcare provider if you have any questions. Children who use RELENZA should always be supervised by an adult who understands how to use RELENZA. Proper use of the DISKHALER to inhale the drug is necessary for safe and effective use of RELENZA.
If you have the flu the usual dose for treatment is 2 inhalations of RELENZA (1 blister per inhalation) twice daily (in the morning and evening) for 5 days. It is important that you begin your treatment with RELENZA as soon as possible from the first appearance of your flu symptoms. Take 2 doses on the first day of treatment whenever possible if there are at least 2 hours between doses.
To reduce the chance of getting the flu, the usual dose is 2 inhalations of RELENZA (1 blister per inhalation) once daily for 10 or 28 days as prescribed by your healthcare provider.
Never share RELENZA with anyone, even if they have the same symptoms. If you feel worse or develop new symptoms during treatment with RELENZA, or if your flu symptoms do not start to get better, stop using the medicine and contact your healthcare provider.
What if I miss a dose?
If you forget to take your medicine at any time, take the missed dose as soon as you remember, except if it is near the next dose (within 2 hours). Then continue to take RELENZA at the usual times. You do not need to take a double dose. If you have missed several doses, inform your healthcare provider and follow the advice given to you.
What are important or common possible side effects of taking RELENZA?
Some patients have had breathing problems while taking RELENZA. This can be very serious and need treatment right away. Most of the patients who had this problem had asthma or chronic obstructive pulmonary disease, but some did not. If you have trouble breathing or have wheezing after your dose of RELENZA, stop taking RELENZA and get medical attention.
In studies, the most common side effects with RELENZA have been headaches; diarrhea; nausea; vomiting; nasal irritation; bronchitis; cough; sinusitis; ear, nose, and throat infections; and dizziness. Other side effects that have been reported, but were not as common, include rashes and allergic reactions, some of which were severe.
People with influenza (the flu), particularly children and adolescents, may be at an increased risk of seizures, confusion, or abnormal behavior early in their illness. These events may occur after beginning RELENZA or may occur when flu is not treated. These events are uncommon but may result in accidental injury to the patient. Therefore, patients should be observed for signs of unusual behavior and a healthcare professional should be contacted immediately if the patient shows any signs of unusual behavior.
This list of side effects is not complete. Your healthcare provider or pharmacist can discuss with you a more complete list of possible side effects with RELENZA. Talk to your healthcare provider promptly about any side effects you have.
Please refer to the section entitled "Important Safety Information About RELENZA" for additional information.
Should I get a flu shot?
RELENZA is not a substitute for a flu shot. You should receive an annual flu shot according to guidelines on immunization practices that your healthcare provider can share with you.
What if I am pregnant or nursing?
If you are pregnant or planning to become pregnant while taking RELENZA, talk to your healthcare provider before taking this medication. RELENZA is normally not recommended for use during pregnancy or nursing, as the effects on the unborn child or nursing infant are unknown.
How and where should I store RELENZA?
RELENZA should be stored at room temperature below 77°F (25°C). RELENZA is not in a childproof container. Keep RELENZA out of the reach of children. Discard the DISKHALER after finishing your treatment.
PATIENT INSTRUCTIONS FOR USE

IMPORTANT: Read Step-by-Step Instructions before using the DISKHALER®.
Be sure to take the dose your healthcare provider has prescribed.
BEFORE YOU START:
Please read the entire Patient Labeling for important information about the effects of RELENZA including the section “Important Safety Information About RELENZA” for information about the risk of breathing difficulties.
If RELENZA is prescribed for a child, dosing should be supervised by an adult who understands how to use RELENZA and has been instructed in its use by a healthcare provider.
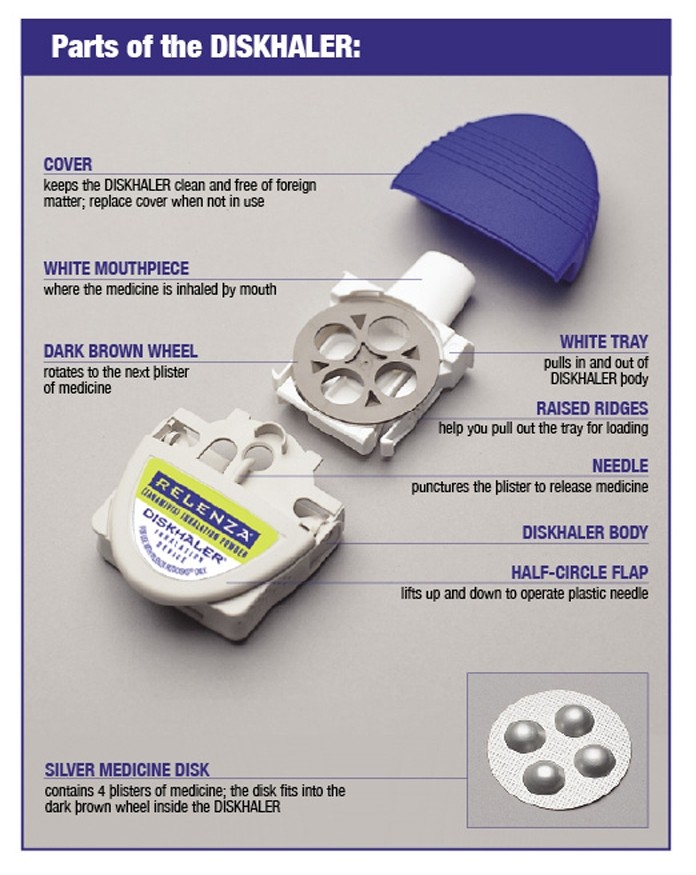
Step-by-step instructions for using the DISKHALER®
Step A: Load the medicine into the DISKHALER
- Start by pulling off the blue cover.
- Always check inside the mouthpiece to make sure it is clear before each use. If foreign objects are in the mouthpiece, they could be inhaled and cause serious harm.
- Pull the white mouthpiece by the edges to extend the white tray all the way.
- Once the white tray is extended all the way, find the raised ridges on each side of it. Press in these ridges, both sides at the same time, and pull the whole white tray out of the DISKHALER body.
- Place one silver medicine disk onto the dark brown wheel, flat side up. The four silver blisters on the underside of the medicine disk will drop neatly into the four holes in the wheel.
- Push in the white tray as far as it will go. Now the DISKHALER is loaded with medicine.
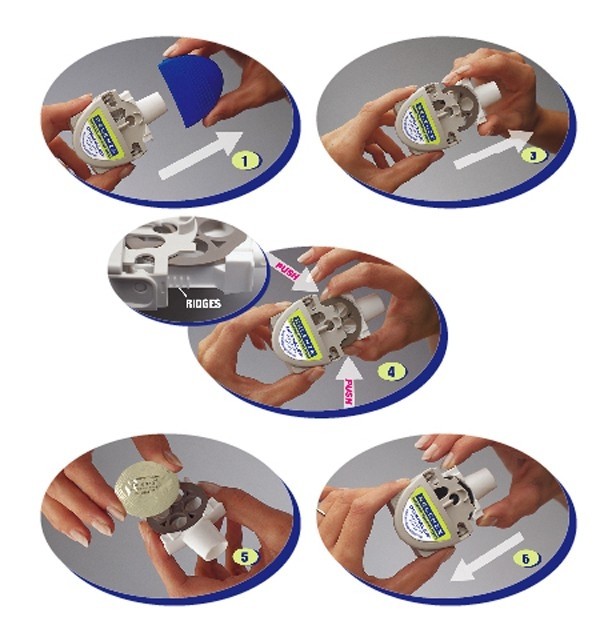
Step B: Puncture the blister
Be sure to keep the DISKHALER level.
The DISKHALER punctures one blister of medicine at a time so you can inhale the right amount. It does not matter which blister you start with. Check to make sure that the silver foil is unbroken.
- Be sure to keep the DISKHALER level so the medicine does not spill out.
- Locate the half-circle flap with the name “RELENZA” on top of the DISKHALER.
- Lift this flap from the outer edge until it cannot go any farther. Flap must be straight up for the plastic needle to puncture both the top and bottom of the silver medicine disk inside.
- Keeping the DISKHALER level, click the flap down into place.
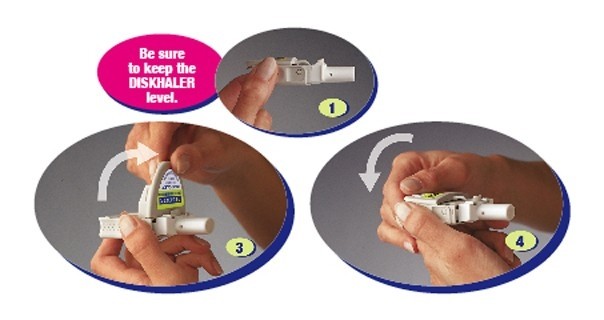
Step C: Inhale
- Before putting the white mouthpiece into your mouth, breathe all the way out (exhale).
Then put the white mouthpiece into your mouth. Be sure to keep the DISKHALER level so the medicine does not spill out.
- Close your lips firmly around the mouthpiece. Be sure not to cover the small holes on either side of it.
- Breathe in through your mouth steadily and as deeply as you can. Your breath pulls the medicine into your airways and lungs.
- Hold your breath for a few seconds to help RELENZA stay in your lungs where it can work.
To take another inhalation, move to the next blister by following Step D below.
Once you’ve inhaled the number of blisters prescribed by your healthcare provider, replace the cover until your next dose.

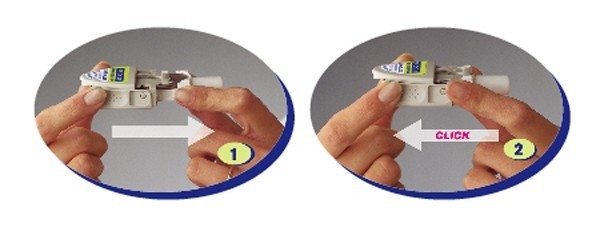
Step D: Move the medicine disk to the next blister
- Pull the mouthpiece to extend the white tray, without removing it.
- Then push it back until it clicks. This pull-push motion rotates the medicine disk to the next blister.
- To take your next inhalation, repeat Steps B and C.
If all four blisters in the medicine disk have been used, you are ready to start a new medicine disk (see Step A). Check to make sure that the silver foil is unbroken each time you are ready to puncture the next blister.
|
IMPORTANT INSTRUCTIONS Read this entire leaflet before using RELENZA. Even if you have had a previous prescription for RELENZA, read this leaflet to see if any information has changed. If you have the flu, the usual dose is 2 inhalations twice daily. To reduce the chance of getting the flu, the usual dose is 2 inhalations once daily. However, you must take the number of inhalations your healthcare provider has prescribed. If you feel worse or develop new symptoms during or after treatment, or if your flu symptoms do not start to improve, stop using the medicine and contact your healthcare provider. Keep out of reach of children. Always check inside the mouthpiece to make sure it is clear before each use. If foreign objects are in the mouthpiece, they could be inhaled and cause serious harm. Always replace the cover after each use. Throw away the DISKHALER after treatment is completed. This DISKHALER is for use only with RELENZA. Do not use the RELENZA DISKHALER device with FLOVENT® (fluticasone propionate) and do not use RELENZA with the FLOVENT DISKHALER device. Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). REMEMBER: This medicine has been prescribed for you by your healthcare provider. DO NOT give this medicine to anyone else. |
RELENZA, FLOVENT, ROTADISK, and DISKHALER are registered trademarks of GlaxoSmithKline.
FLUMIST is a registered trademark of MedImmune, Inc.
PRINCIPAL DISPLAY PANEL
NDC 54868-4377-0

RELENZA®
(ZANAMIVIR) INHALATION POWDER
5 ROTADISKS® each containing 4 blisters
5 mg
FOR ORAL INHALATION ONLY
Each blister contains 5 mg zanamivir with lactose.
Store at 25oC (77oF); excursions permitted to 15o to 30oC (59o to 86oF) (see USP Controlled Room Temperature).
Rx only
See prescribing information for dosage information.
RELENZAzanamivir POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||