Relistor
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RELISTOR safely and effectively. See full prescribing information for RELISTOR. RELISTOR (methylnaltrexone bromide) Subcutaneous InjectionInitial U.S. Approval: 2008RECENT MAJOR CHANGES General Dosing Information (2.1) Dosing (2.2) Use in Patients with Severe Renal Impairment (2.3) Administration and Storage (2.4) Gastrointestinal Perforation (5.1) Severe or Persistent Diarrhea (5.2) [08/2013] [08/2013] [08/2013] [08/2013] [08/2013] [08/2013] INDICATIONS AND USAGERELISTOR is indicated for the treatment of opioid-induced constipation in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient. Use of RELISTOR beyond four months has not been studied. ( 1 ) DOSAGE AND ADMINISTRATIONRELISTOR is administered as a subcutaneous injection. The usual schedule is one dose every other day, as needed, but no more frequently than one dose in a 24-hour period. ( 2.2 )The recommended dose of RELISTOR is 8 mg for patients weighing 38 to less than 62 kg or 12 mg for patients weighing 62 to 114 kg. Patients whose weights fall outside of these ranges should be dosed at 0.15 mg/kg. See the table below to determine the correct injection volume. ( 2.2) Patient Weight Injection Volume Dose Less than 38 kg See below* 0.15 mg/kg 38 kg to less than 62 kg 0.4 mL 8 mg 62 kg to 114 kg 0.6 mL 12 mg More than 114 kg See below* 0.15 mg/kg * The injection volume for these patients should be calculated using one of the following ( 2.2 ): Multiply the patient weight in kilograms by 0.0075 and round up the volume to the nearest 0.1 mL. Only patients requiring an 8 mg or 12 mg dose should be prescribed pre-filled syringes ( 2.2 , 3 ).In patients with severe renal impairment (creatinine clearance less than 30 mL/min), dose reductions of RELISTOR by one half is recommended. (8.6) DOSAGE FORMS AND STRENGTHSRELISTOR is available in the following dosage forms: Single-use vial containing 12 mg/0.6 mL solution for subcutaneous injection, for use with a 27 gauge x ½-inch needle and 1 mL syringe Single-use vial containing 12 mg/0.6 mL solution for subcutaneous injection with one 1 mL syringe with retractable 27 gauge x ½-inch needle, two alcohol swabs. Single-use pre-filled syringe containing 8 mg/0.4 mL solution for subcutaneous injection. Single-use pre-filled syringe containing 12 mg/0.6 mL solution for subcutaneous injection. CONTRAINDICATIONS RELISTOR is contraindicated in patients with known or suspected mechanical gastrointestinal obstruction. ( 4 ) WARNINGS AND PRECAUTIONS Rare cases of gastrointestinal (GI) perforation have been reported in advanced illness patients. Use RELISTOR with caution in patients with known or suspected lesions of the GI tract. ( 5.1 ) If severe or persistent diarrhea occurs during treatment, advise patients to discontinue therapy with RELISTOR and consult their physician. ( 5.2 ) Side EffectsThe most common (≥5%) adverse reactions reported with RELISTOR are abdominal pain, flatulence, nausea, dizziness, diarrhea and hyperhidrosis. ( 6.1 ) To report SUSPECTED ADVERSE REACTIONS, contact Salix Pharmaceuticals Inc. at 1-800-508-0024 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONSIn an in vivo study Relistor did not significantly affect the metabolism of the CYP2D6 substrate, dextromethorphan. In vitro methylnaltrexone did not significantly inhibit or induce cytochrome P450 (CYP) isozymes including CYP 1A2, 2A6, 2B6, 2C9, 2C19, or 3A4 ( 7.1 )USE IN SPECIFIC POPULATIONSPediatric Use: Safety and efficacy of RELISTOR have not been established in pediatric patients. ( 8.4 )
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 RELISTOR INDICATIONS AND USAGE
- 2 RELISTOR DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 RELISTOR CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 RELISTOR ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 RELISTOR DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PATIENT INFORMATION
- Patient Instructions for Use of RELISTOR PRE-FILLED SYRINGE
- Patient Instructions for Use of RELISTORVIAL AND SYRINGE WITH RETRACTABLE NEEDLE IN TRAY
- Patient Instructions for Use of RELISTOR® VIAL AND STANDARD SYRINGE AND NEEDLE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - VIAL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - TRAY
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 8 mg/0.4 mL - SYRINGE LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 8 mg/0.4 mL - SYRINGE LIDDING
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 8 mg/0.4 mL - CARTON
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LIDDING
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LABEL - SINGLE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LIDDING - SINGLE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON - SINGLE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LABEL - SINGLE - SAMPLE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LIDDING - SINGLE - SAMPLE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON - SINGLE - SAMPLE
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
RELISTOR® is indicated for the treatment of opioid-induced constipation in patients with advanced illness who are receiving palliative care, when response to laxative therapy has not been sufficient.
Limitation of use: Use of RELISTOR beyond four months has not been studied in the advanced illness population.
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
RELISTOR is for subcutaneous use only.
2.2 Dosing
For adult patients with opioid-induced constipation and advanced illness, the usual schedule is one dose every other day, as needed, but no more frequently than one dose in a 24-hour period [see Clinical Studies ( 14 )].
The recommended dose of RELISTOR is 8 mg subcutaneously for opioid-induced constipation and advanced illness adult patients weighing 38 kg to less than 62 kg or 12 mg subcutaneously for patients weighing 62 kg to 114 kg. Adult patients whose weight falls outside of these ranges should be dosed at 0.15 mg/kg. See Table 1 to determine the correct injection volume. The pre-filled syringe is designed to deliver a fixed dose; therefore, adult patients requiring dosing calculated on a mg/kg basis should not be prescribed pre-filled syringes.
|
Table 1: Weight of Adult Patient with Opioid-Induced Constipation and Advanced Illness |
Injection Volume |
Subcutaneous Dose |
|
Less than 38 kg |
See below* |
0.15 mg/kg |
|
38 kg to less than 62 kg |
0.4 mL |
8 mg |
|
62 kg to 114 kg |
0.6 mL |
12 mg |
|
More than 114 kg |
See below* |
0.15 mg/kg |
* The injection volume for these patients should be calculated using the following method:
Multiply the patient weight in kilograms by 0.0075 and round up the volume to the nearest 0.1 mL.
2.3 Use in Patients with Severe Renal Impairment
In adult patients with severe renal impairment (creatinine clearance less than 30 mL/min as estimated by Cockcroft-Gault), dose reduction of RELISTOR by one-half is recommended [see Use in Specific Populations ( 8.6 )]. No dosage adjustment is recommended for adult patients with mild to moderate renal impairment.
The pre-filled syringe is designed to deliver a fixed dose; therefore, adult patients with severe renal impairment should only be prescribed single-use vials to ensure correct dosing.
2.4 Administration and Storage
RELISTOR is a sterile, clear, and colorless to pale yellow aqueous solution. Inspect parenteral drug product visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the vial if any of these are present.
Inject RELISTOR subcutaneously in the upper arm, abdomen or thigh. Do not inject at the same spot each time (rotate injection sites).
Single-use Vials
Once drawn into the 1 mL syringe with a 27-gauge x ½-inch needle, if immediate administration is not possible, store at ambient room temperature and administer within 24 hours. Discard any unused portion that remains in the vial. Advise patients concerning proper training in subcutaneous technique.
Single-use Pre-filled Syringes
Only adult patients requiring an 8 mg or 12 mg dose should be prescribed pre-filled syringes. Do not remove the pre-filled syringe from the tray until ready to administer.
3 DOSAGE FORMS AND STRENGTHS
- Single-use vial containing 12 mg/0.6 mL solution for subcutaneous injection, for use with a 27 gauge x ½-inch needle and 1 mL syringe
- Single-use vial containing 12 mg/0.6 mL solution for subcutaneous injection, with one 1 mL syringe with retractable 27 gauge x ½-inch needle, two alcohol swabs
- Single-use pre-filled syringe containing 8 mg/0.4 mL solution for subcutaneous injection, with a 29-gauge x ½-inch fixed needle and a needle guard
- Single-use pre-filled syringe containing 12 mg/0.6 mL solution for subcutaneous injection, with a 29-gauge x ½-inch fixed needle and a needle guard
4 CONTRAINDICATIONS
RELISTOR is contraindicated in patients with known or suspected mechanical gastrointestinal obstruction.
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Perforation
Cases of gastrointestinal (GI) perforation have been reported in adult patients with opioid-induced constipation and advanced illness with conditions that may be associated with localized or diffuse reduction of structural integrity in the wall of the GI tract (i.e., cancer, peptic ulcer, Ogilvie’s syndrome). Perforations have involved varying regions of the GI tract (e.g., stomach, duodenum, or colon).
Use RELISTOR with caution in patients with known or suspected lesions of the GI tract. Advise patients to discontinue therapy with RELISTOR and promptly notify their physician if they develop severe, persistent, or worsening abdominal symptoms.
5.2 Severe or Persistent Diarrhea
If severe or persistent diarrhea occurs during treatment, advise patients to discontinue therapy with RELISTOR and consult their physician.
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of RELISTOR was evaluated in two, double-blind, placebo-controlled trials in patients with advanced illness receiving palliative care: Study 1 included a single‑dose, double‑blind, placebo-controlled period, whereas Study 2 included a 14-day multiple dose, double-blind, placebo-controlled period [see Clinical Studies ( 14 )]. The majority of patients had a primary diagnosis of incurable cancer; other primary diagnoses included end-stage COPD/emphysema, cardiovascular disease/heart failure, Alzheimer"s disease/dementia, HIV/AIDS, or other advanced illnesses. Patients were receiving opioid therapy (median daily baseline oral morphine equivalent dose = 172 mg), and had opioid‑induced constipation (either <3 bowel movements in the preceding week or no bowel movement for 2 days). Both the methylnaltrexone bromide and placebo patients were on a stable laxative regimen for at least 3 days prior to study entry and continued on their regimen throughout the study.
The most common (≥5%) adverse reactions in patients receiving RELISTOR are shown in Table 2 below.
|
Table
2 |
||
|
Adverse Reaction |
RELISTOR
|
Placebo
|
|
Abdominal Pain |
47 (28.5%) |
12 (9.8%) |
|
Flatulence |
22 (13.3%) |
7 (5.7%) |
|
Nausea |
19 (11.5%) |
6 (4.9%) |
|
Dizziness |
12 (7.3%) |
3 (2.4%) |
|
Diarrhea |
9 (5.5%) |
3 (2.4%) |
|
Hyperhidrosis |
11 (6.7%) |
8 (6.5%) |
* Doses: 0.075, 0.15, and 0.30 mg/kg/dose
The rates of discontinuation due to adverse events during the double-blind placebo controlled clinical trials (Study 1 and Study 2) were comparable between RELISTOR (1.2%) and placebo (2.4%).
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of RELISTOR. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting or causal connection to RELISTOR, or a combination of these factors.
Gastrointestinal
Perforation, cramping, vomiting
General Disorders and Administrative Site Disorders
Diaphoresis, flushing, malaise, pain. Cases of opioid withdrawal have been reported.
7 DRUG INTERACTIONS
7.1 Drugs Metabolized by Cytochrome P450 Isozymes
In healthy subjects, a subcutaneous dose of 0.30 mg/kg of methylnaltrexone did not significantly affect the metabolism of dextromethorphan, a CYP2D6 substrate.
In vitro methylnaltrexone did not significantly inhibit or induce the activity of cytochrome P450 (CYP) isozymes CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, or CYP3A4.
In vitro, methylnaltrexone did not induce the enzymatic activity of CYP2E1.
7.2 Drugs Renally Excreted
Methylnaltrexone is actively secreted in the kidney. The potential of drug interactions between methylnaltrexone bromide and other drugs that are inhibitors of transporters in the kidney has not been fully investigated [see Pharmacokinetics ( 12.3 )].
7.3 Cimetidine
Cimetidine given 400 mg three times daily did not significantly affect the systemic exposure to methylnaltrexone. The effect of a higher cimetidine dose (e.g., 800 mg) on the systemic exposure of methylnaltrexone has not been evaluated.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies have been performed in pregnant rats at intravenous doses up to about 14 times the recommended maximum human subcutaneous dose of 0.3 mg/kg based on the body surface area and in pregnant rabbits at intravenous doses up to about 17 times the recommended maximum human subcutaneous dose based on the body surface area and have revealed no evidence of impaired fertility or harm to the fetus due to methylnaltrexone bromide. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, methylnaltrexone bromide should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
Effects of RELISTOR on mother, fetus, duration of labor, and delivery are unknown. There were no effects on the mother, labor, delivery, or on offspring survival and growth in rats following subcutaneous injection of methylnaltrexone bromide at dosages up to 25 mg/kg/day.
8.3 Nursing Mothers
Results from an animal study using [3H]-labeled methylnaltrexone bromide indicate that methylnaltrexone bromide is excreted via the milk of lactating rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when RELISTOR is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of RELISTOR have not been established in pediatric patients.
8.5 Geriatric Use
In the phase 2 and 3 double-blind studies, a total of 77 (24%) patients aged 65-74 years (54 methylnaltrexone bromide, 23 placebo) and a total of 100 (31.2%) patients aged 75 years or older (61 methylnaltrexone bromide, 39 placebo) were enrolled. Pharmacokinetics of methylnaltrexone was similar between the elderly (mean age 72 years old) and young adults (mean age 30 years old). No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Based on pharmacokinetic data, and safety and efficacy data from controlled clinical trials, no dose adjustment based on age is recommended.
8.6 Renal Impairment
No dose adjustment is required in patients with mild or moderate renal impairment. Dose‑reduction by one half is recommended in patients with severe renal impairment (creatinine clearance less than 30 mL/min as estimated by Cockcroft-Gault).
In a study of volunteers with varying degrees of renal impairment receiving a single dose of 0.30 mg/kg methylnaltrexone bromide, renal impairment had a marked effect on the renal excretion of methylnaltrexone bromide. Severe renal impairment decreased the renal clearance of methylnaltrexone bromide by 8- to 9-fold and resulted in a 2-fold increase in total methylnaltrexone bromide exposure (AUC). Cmax was not significantly changed. No studies were performed in patients with end-stage renal impairment requiring dialysis.
8.7 Hepatic Impairment
No dose adjustment is required for patients with mild or moderate hepatic impairment. The effect of severe hepatic impairment on the pharmacokinetics of methylnaltrexone has not been studied.
10 OVERDOSAGE
During clinical trials of RELISTOR administered subcutaneously, no cases of methylnaltrexone bromide overdose were reported. A study of healthy volunteers noted orthostatic hypotension associated with a dose of 0.64 mg/kg administered as an intravenous bolus.
Signs or symptoms of orthostatic hypotension should be monitored, and treatment should be initiated, as appropriate.
11 DESCRIPTION
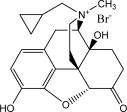
RELISTOR (methylnaltrexone bromide) injection, a peripherally-acting mu-opioid receptor antagonist, is a sterile, clear and colorless to pale yellow aqueous solution. The chemical name for methylnaltrexone bromide is (R)-N-(cyclopropylmethyl) noroxymorphone methobromide. The molecular formula is C21H26NO4Br, and the molecular weight is 436.36.
Each 3 mL vial contains 12 mg of methylnaltrexone bromide in 0.6 mL of water. The excipients are 3.9 mg sodium chloride USP, 0.24 mg edetate calcium disodium USP, and 0.18 mg glycine hydrochloride. During manufacture, the pH may have been adjusted with hydrochloric acid and/or sodium hydroxide.
Each 8 mg/0.4 mL pre-filled syringe (1 mL syringe) contains 8 mg of methylnaltrexone bromide in 0.4 mL of water. The excipients are 2.6 mg sodium chloride USP, 0.16 mg edetate calcium disodium USP, and 0.12 mg glycine hydrochloride.
Each 12 mg/0.6 mL pre-filled syringe (1 mL syringe) contains 12 mg of methylnaltrexone bromide in 0.6 mL of water. The excipients are 3.9 mg sodium chloride USP, 0.24 mg edetate calcium disodium USP, and 0.18 mg glycine hydrochloride.
The structural formula is:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methylnaltrexone is a selective antagonist of opioid binding at the mu-opioid receptor. As a quaternary amine, the ability of methylnaltrexone to cross the blood-brain barrier is restricted. This allows methylnaltrexone to function as a peripherally-acting mu‑opioid receptor antagonist in tissues such as the gastrointestinal tract, thereby decreasing the constipating effects of opioids without impacting opioid-mediated analgesic effects on the central nervous system.
12.2 Pharmacodynamics
Effect on Cardiac Repolarization
In a randomized, double-blind placebo- and (open-label) moxifloxacin-controlled 4‑period crossover study, 56 healthy subjects were administered methylnaltrexone bromide 0.3 mg/kg and methylnaltrexone bromide 0.64 mg/kg by intravenous infusion over 20 minutes, placebo, and a single oral dose of moxifloxacin. At both the 0.3 mg/kg and 0.64 mg/kg methylnaltrexone bromide doses, no significant effect on the QTc interval was detected.
12.3 Pharmacokinetics
Absorption
Following subcutaneous administration, methylnaltrexone achieved peak concentrations (Cmax) at approximately 0.5 hours. Across the range of doses from 0.15 mg/kg to 0.50 mg/kg, mean Cmax and area under the plasma concentration-time curve (AUC) increased in a dose-proportional manner. There was no accumulation of methylnaltrexone following once-daily subcutaneous dosing of methylnaltrexone bromide 12 mg for seven consecutive days in healthy subjects.
|
Table 3: Pharmacokinetic Parameters of Methylnaltrexone Following Subcutaneous Doses |
|||
|
Parameter |
0.15 mg/kg single dose |
12 mg single dose |
12 mg at steady-state |
|
Cmax (ng/mL) i) |
117 (32.7) |
140 (35.6) |
119 (27.2) |
|
tmax (hr) ii) |
0.5 (0.25-0.75) |
0.25 (0.25-0.5) |
0.25 (0.25-0.5) |
|
AUC24 (ng·hr/mL) i) |
175 (36.6) |
218 (28.3) |
223 (28.2) |
i) Expressed as mean (SD).
ii) Expressed as median (range).
Distribution
The steady-state volume of distribution (Vss) of methylnaltrexone is approximately 1.1 L/kg. The fraction of methylnaltrexone bound to human plasma proteins is 11.0% to 15.3%, as determined by equilibrium dialysis.
Metabolism
In a mass balance study, approximately 44% of the administered radioactivity was recovered in the urine over 24 hours with 5 distinct metabolites and none of the detected metabolites was in amounts over 6% of administered radioactivity. Conversion to methyl-6-naltrexol isomers (5% of total) and methylnaltrexone sulfate (1.3% of total) appear to be the primary pathways of metabolism. N‑demethylation of methylnaltrexone to produce naltrexone is not significant.
After 12 mg once daily dosing the mean AUC0-24 ratio of metabolites to methylnaltrexone at steady-state was 30%, 19%, and 9% for methylnaltrexone sulfate, methyl-6α-naltrexol, and methyl-6β-naltrexol, respectively. Methyl-6α-naltrexol, and methyl-6β-naltrexol were active mu-opioid receptor antagonists and methylnaltrexone sulfate is a weak mu-opioid receptor antagonist.
Methylnaltrexone is conjugated by sulfotransferase SULT1E1 and SULT2A1 isoforms to methylnaltrexone sulfate. Conversion to methyl-6-naltrexol isomers is mediated by aldo-keto reductase 1C enzymes.
Excretion
After intravenous administration, approximately half of the dose was excreted in the urine (53.6%) and 17.3% of administered dose was excreted in the feces up to 168 hours postdose. Methylnaltrexone is excreted primarily as the unchanged drug in the urine and feces. The terminal half-life (t1/2) is approximately 8 hours. Active renal secretion of methylnaltrexone is suggested by renal clearance of methylnaltrexone that is approximately 4-5 fold higher than creatinine clearance.
Specific Populations
Geriatric
A study was conducted to characterize the pharmacokinetics of methylnaltrexone after single dose of 24 mg methylnaltrexone via intravenous infusion over 20 min in healthy adults between 18 and 45 years of age and in healthy adults aged 65 years and older. In elderly subjects, mean clearance was about 20% lower (56 L/h versus 70 L/h) and AUC∞ was 26% higher than in subjects between 18 and 45 years of age.
Renal impairment
In a study of volunteers with varying degrees of renal impairment receiving a single dose of 0.30 mg/kg methylnaltrexone bromide, renal impairment had a marked effect on the renal excretion of methylnaltrexone. Severe renal impairment decreased the renal clearance of methylnaltrexone by 8- to 9-fold and resulted in a 2-fold increase in total methylnaltrexone exposure (AUC). Mean Cmax was not significantly changed.
Hepatic impairment
The effect of mild and moderate hepatic impairment on the systemic exposure to methylnaltrexone has been studied in 8 subjects each, with Child-Pugh Class A and B, compared to healthy subjects. Results showed no meaningful effect of hepatic impairment on the AUC or Cmax of methylnaltrexone.
Drug Interactions
In vitro studies suggested that methylnaltrexone was a substrate of Organic Cation Transporter 1 but not a substrate of Organic Anion Transporter 1 or of P-glycoprotein.
Cimetidine
A clinical drug interaction study in healthy adult subjects evaluated the effects of cimetidine, a drug that inhibits the active renal secretion of organic cations, on the pharmacokinetics of methylnaltrexone (24 mg administered as an IV infusion over 20 minutes). A single dose of methylnaltrexone was administered before cimetidine dosing and with the last dose of cimetidine (400 mg every 8 hours for 6 days). Mean Cmax and AUC of methylnaltrexone increased by 10% with concomitant cimetidine administration. The renal clearance of methylnaltrexone decreased about 40%.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two-year oral carcinogenicity studies have been conducted with methylnaltrexone in CD-1 mice at doses up to 200 mg/kg/day (about 108 times the recommended human dose of 0.15 mg/kg based on body surface area) in males and 400 mg/kg/day (about 216 times the recommended human dose of 0.15 mg/kg based on body surface area) in females and in Sprague Dawley rats at oral doses up to 300 mg/kg/day (about 324 times the recommended human dose of 0.15 mg/kg based on body surface area). Oral administration of methylnaltrexone for 104 weeks did not produce tumors in mice and rats.
Mutagenesis
Methylnaltrexone bromide was negative in the Ames test, chromosome aberration tests in Chinese hamster ovary cells and human lymphocytes, in the mouse lymphoma cell forward mutation tests and in the in vivo mouse micronucleus test.
Impairment of Fertility
Methylnaltrexone bromide at subcutaneous doses up to 150 mg/kg/day (about 81 times the recommended maximum human subcutaneous dose based on the body surface area) was found to have no adverse effect on fertility and reproductive performance of male and female rats.
13.2 Animal Toxicology and/or Pharmacology
In an in vitro human cardiac potassium ion channel (hERG) assay, methylnaltrexone bromide caused concentration-dependent inhibition of hERG current (1%, 12%, 13% and 40% inhibition at 30, 100, 300 and 1000 μM concentrations, respectively). Methylnaltrexone bromide had a hERG IC50 of > 1000 μM. In isolated dog Purkinje fibers, methylnaltrexone bromide caused prolongations in action potential duration (APD). The highest tested concentration (10 μM) in the dog Purkinje fiber study was about 18 and 37 times the Cmax at human subcutaneous (SC) doses of 0.3 and 0.15 mg/kg, respectively. In isolated rabbit Purkinje fibers, methylnaltrexone bromide (up to 100 μM) did not have an effect on APD, compared to vehicle control. The highest methylnaltrexone bromide concentration (100 μM) tested was about 186 and 373 times the human Cmax at SC doses of 0.3 and 0.15 mg/kg, respectively. In anesthetized dogs, methylnaltrexone bromide caused decreases in blood pressure, heart rate, cardiac output, left ventricular pressure, left ventricular end diastolic pressure, and +dP/dt at ≥ 1 mg/kg. In conscious dogs, methylnaltrexone bromide caused a dose-related increase in QTc interval. After a single intravenous dosage of 20 mg/kg to beagle dogs, predicted Cmax and AUC values were approximately 482 and 144 times, respectively, the exposure at human SC dose of 0.15 mg/kg and 241 times and 66 times, respectively, the exposure at a human SC dose of 0.3 mg/kg. In conscious guinea pigs, methylnaltrexone caused mild prolongation of QTc (4% over baseline) at 20 mg/kg, intravenous. A thorough QTc assessment was conducted in humans [see Clinical Pharmacology (12.2)].
In juvenile rats administered intravenous methylnaltrexone bromide for 13 weeks, adverse clinical signs such as convulsions, tremors and labored breathing occurred at dosages of 3 and 10 mg/kg/day (about 3.2 and 11 times, respectively, the recommended human dose of 0.15 mg/kg based on the body surface area). Similar adverse clinical signs were seen in adult rats at 20 mg/kg/day (about 22 times the recommended human dose of 0.15 mg/kg based on the body surface area). Juvenile rats were found to be more sensitive to the toxicity of methylnaltrexone bromide when compared to adults. The no observed adverse effect levels (NOAELs) in juvenile and adult rats were 1 and 5 mg/kg/day, respectively (about 1.1 and 5.4 times respectively, the recommended human dose of 0.15 mg/kg based on the body surface area).
In juvenile dogs administered intravenous methylnaltrexone bromide for 13 weeks, juvenile dogs had a toxicity profile similar to adult dogs. Following intravenous administration of methylnaltrexone bromide for 13 weeks, decreased heart rate (13.2 % reduction compared to pre-dose) in juvenile dogs and prolonged QTc interval in juvenile (9.6% compared to control) and adult (up to 15% compared to control) dogs occurred at 20 mg/kg/day (about 72 times the recommended human subcutaneous doses of 0.15 mg/kg based on the body surface area). Clinical signs consistent with effects on the CNS (including tremors and decreased activity) occurred in both juvenile and adult dogs. The NOAELs in juvenile and adult dogs were 5 mg/kg/day (about 18 times the recommended human subcutaneous doses of 0.15 mg/kg based on the body surface area).
14 CLINICAL STUDIES
The efficacy and safety of RELISTOR in the treatment of opioid-induced constipation in advanced illness patients receiving palliative care was demonstrated in two randomized, double‑blind, placebo‑controlled studies. In these studies, the median age was 68 years (range 21-100); 51% were females. The majority of patients had a primary diagnosis of incurable cancer; other primary diagnoses included end-stage COPD/emphysema, cardiovascular disease/heart failure, Alzheimer's disease/dementia, HIV/AIDS, or other advanced illnesses. Prior to screening, patients had been receiving palliative opioid therapy (median daily baseline oral morphine equivalent dose = 172 mg), and had opioid‑induced constipation (either <3 bowel movements in the preceding week or no bowel movement for >2 days). Patients were on a stable opioid regimen ≥ 3 days prior to randomization (not including PRN or rescue pain medication) and received their opioid medication during the study as clinically needed. Patients maintained their regular laxative regimen for at least 3 days prior to study entry, and throughout the study. Rescue laxatives were prohibited from 4 hours before to 4 hours after taking an injection of study medication.
Study 1 compared a single, double-blind, subcutaneous dose of RELISTOR 0.15 mg/kg, or RELISTOR 0.3 mg/kg versus placebo. The double-blind dose was followed by an open-label 4‑week dosing period, where RELISTOR could be used as needed, no more frequently than 1 dose in a 24 hour period. Throughout both study periods, patients maintained their regular laxative regimen. A total of 154 patients(47 RELISTOR 0.15 mg/kg, 55 RELISTOR 0.3 mg/kg, 52 placebo) were enrolled and treated in the double-blind period. The primary endpoint was the proportion of patients with a rescue-free laxation within 4 hours of the double-blind dose of study medication. RELISTOR-treated patients had a significantly higher rate of laxation within 4 hours of the double-blind dose (62% for 0.15 mg/kg and 58% for 0.3 mg/kg) than did placebo‑treated patients (14%); p < 0.0001 for each dose versus placebo (Figure 1).
Study 2 compared double-blind, subcutaneous doses of RELISTOR given every other day for 2 weeks versus placebo. Patients received opioid medication ≥ 2 weeks prior to receiving study medication. During the first week (days 1, 3, 5, 7) patients received either 0.15 mg/kg RELISTOR or placebo. In the second week the patient's assigned dose could be increased to 0.30 mg/kg if the patient had 2 or fewer rescue-free laxations up to day 8. At any time, the patient's assigned dose could be reduced based on tolerability. Data from 133 (62 RELISTOR, 71 placebo) patients were analyzed. There were 2 primary endpoints: proportion of patients with a rescue-free laxation within 4 hours of the first dose of study medication and proportion of patients with a rescue-free laxation within 4 hours after at least 2 of the first 4 doses of study medication. RELISTOR-treated patients had a higher rate of laxation within 4 hours of the first dose (48%) than placebo-treated patients (16%); p < 0.0001 (Figure 1). RELISTOR-treated patients also had significantly higher rates of laxation within 4 hours after at least 2 of the first 4 doses (52%) than did placebo-treated patients (9%); p < 0.0001. In both studies, in approximately 30% of patients, laxation was reported within 30 minutes of a dose of RELISTOR.
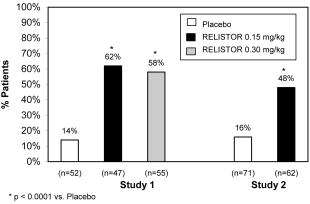
In both studies, there was no evidence of differential effects of age or gender on safety or efficacy. No meaningful subgroup analysis could be conducted on race because the study population was predominantly Caucasian (88%).
Durability of Response
Durability of response was explored in Study 2 and the laxation response rate was consistent from dose 1 through dose 7 over the course of the 2-week, double-blind period.
The efficacy and safety of methylnaltrexone bromide was also demonstrated in open-label treatment administered from Day 2 through Week 4 in Study 1, and in two open-label extension studies (Study 1EXT and Study 2EXT) in which RELISTOR was given as needed for up to 4 months. During open-label treatment, patients maintained their regular laxative regimen. A total of 136, 21, and 82 patients received at least 1 open-label dose in studies 1, 1EXT, and 2EXT, respectively. Laxation response was also explored in this open-label setting and appeared to be maintained over the course of 3 to 4 months of open-label treatment.
Opioid Use and Pain Scores
No relationship between baseline opioid dose and laxation response in methylnaltrexone bromide-treated patients was identified in exploratory analyses of these studies. In addition, median daily opioid dose did not vary meaningfully from baseline in either RELISTOR-treated patients or in placebo‑treated patients. There were no clinically relevant changes in pain scores from baseline in either the methylnaltrexone bromide or placebo-treated patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
|
NDC |
PACK SIZE |
CONTENTS |
|
65649-551-02 |
1 vial per carton |
one 12 mg/0.6 mL single-use vial |
|
65649-553-05 |
7 trays per kit |
Each tray contains: |
|
65649-552-04 |
7 pre-filled syringes per carton |
seven 8 mg/0.4 mL single-use pre-filled syringes with needle guard system |
|
65649-551-03 |
7 pre-filled syringes per carton |
seven 12 mg/0.6 mL single-use pre-filled syringes with needle guard system |
|
65649-551-07 |
1 pre-filled syringe per carton |
one 12mg/0.6 mL single-use pre-filled syringe with needle guard system |
16.1 Storage
RELISTOR should be stored at 20˚C to 25ºC (68˚F to 77ºF); excursions permitted to 15˚C to 30°C (59˚F to 86°F) [see USP Controlled Room Temperature]. Do not freeze. Protect from light.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (PatientInformation and Instructions for Use)
- Instruct patients not to continue taking RELISTOR and to promptly notify their physician if they experience severe, persistent, or worsening abdominal symptoms because these could be symptoms of gastrointestinal perforation [see Warnings and Precautions (5.1)].
- Instruct patients not to continue taking RELISTOR if they experience severe or persistent diarrhea. Inform patients that common side effects of RELISTOR include abdominal pain, flatulence, nausea, dizziness, and diarrhea.
- Advise patients to be within close proximity to toilet facilities once the drug is administered.
- Instruct patients with opioid-induced constipation and advanced illness to administer one dose subcutaneously every other day, as needed, but no more frequently than one dose in a 24-hour period.
- Instruct patients to discontinue RELISTOR if they stop taking their opioid pain medication.
- Instruct patients to use the RELISTOR single-use vial with a 27 gauge x ½-inch needle and 1 mL syringe.
PATIENT INFORMATION
Patient Information
RELISTOR®
(rel - i – store)
(methylnaltrexone bromide)
Subcutaneous Injection
Read this Patient Information that comes with RELISTOR before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is RELISTOR?
RELISTOR is a prescription medicine used to treat constipation that is caused by prescription pain medicines, called opioids, in patients receiving supportive care for their advanced illness, when other medicines for constipation, called laxatives, have not worked well enough.
It is not known if RELISTOR is safe and effective if used for longer than 4 months in people with advanced illness.
It is not known if RELISTOR is safe and effective in children.
Who should not use RELISTOR?
Do not use RELISTOR if you have or may have a blockage in your intestines called a mechanical bowel obstruction. Symptoms of this blockage are vomiting, stomach pain, and swelling of your stomach-area (abdomen). Talk to your healthcare provider if you have any of these symptoms before using RELISTOR.
What should I tell my healthcare provider before using RELISTOR?
Before you start using RELISTOR, tell your healthcare provider if you:
- have kidney problems
- have or had cancer of the stomach or intestine
- have or had a stomach ulcer
- have had a blockage in your intestine
- have any other medical condition
- are pregnant or plan to become pregnant. It is not known if RELISTOR can harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if RELISTOR passes into your breast milk.
Tell your healthcare provider about all medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Continue taking your other medicines for constipation unless your healthcare provider tells you to stop taking them.
How should I use RELISTOR?
- RELISTOR is injected under the skin (subcutaneous injection) of the upper arm, abdomen, or thigh.
- Inject RELISTOR exactly as your healthcare provider tells you.
- RELISTOR is usually used every other day. Do not inject more than one dose of RELISTOR in a 24-hour period.
- Stay close to a toilet after using RELISTOR.
- Stop using RELISTOR if you stop taking your prescription opioid pain medicine.
- If you inject more RELISTOR than prescribed, talk to your healthcare provider right away.
See the detailed Instructions for Use that comes with RELISTOR for information about how to prepare and inject RELISTOR, and properly throw away (dispose of) used needles and syringes the right way.
What are the possible side effects of RELISTOR?
RELISTOR can cause serious side effects, including:
- Tear in your stomach or intestinal wall (perforation).
Stop using RELISTOR and call your healthcare provider right away if you develop swelling or pain in your stomach-area (abdomen) that is severe, gets worse, or that does not go away, nausea or vomiting that does not go away, or if you vomit blood or have black sticky stools.
- Diarrhea that is severe or that will not go away. Stop using RELISTOR and call your healthcare provider if you get diarrhea that is severe or that does not go away during treatment with RELISTOR.
The most common side effects of RELISTOR include:
- stomach-area (abdomen) pain
- gas
- nausea
- dizziness
- diarrhea
- sweating
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of RELISTOR.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
How should I store RELISTOR?
- Store RELISTOR vials and pre-filled syringes at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze RELISTOR.
- Keep RELISTOR away from light until you are ready to use it.
- If RELISTOR has been drawn into a syringe and you are unable to use the medicine right away, keep the syringe at room temperature for up to 24 hours. The syringe does not need to be kept away from light during the 24-hour period.
Keep RELISTOR and all medicines, needles and syringes out of the reach of children.
General information about RELISTOR
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use RELISTOR for a condition for which it was not prescribed. Do not give RELISTOR to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about RELISTOR. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about RELISTOR that is written for health professionals.
For more information, go to WWW.RELISTOR.COM.
What are the ingredients in RELISTOR?
Active ingredient: methylnaltrexone bromide
Inactive ingredients: sodium chloride, edetate calcium disodium USP, glycine hydrochloride.
During manufacture, the pH may have been adjusted with hydrochloric acid and/or sodium hydroxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:

Salix Pharmaceuticals, Inc.
Raleigh, NC 27615
Under license from:

Progenics Pharmaceuticals, Inc.
Tarrytown, NY 10591
Revised: AUG 2013
Product protected by U.S. Patent Nos. 6,559,158, 8,247,425, and 8,420,663.
Please see www.salix.com for patent information
Patient Instructions for Use of RELISTOR PRE-FILLED SYRINGE
Instructions for Use
RELISTOR® (rel-i-store)
(methylnaltrexone bromide)
Pre-filled Syringe
Read this Instructions for Use before you start using RELISTOR and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
The following instructions explain how to prepare and give an injection of RELISTOR the right way, when using a pre-filled syringe of RELISTOR.
Important information:
- Do not use a RELISTOR pre-filled syringe and attached needle more than one time, even if there is medicine left in the syringe. See Step 4 “Dispose of used pre-filled syringes and needles.”
- Safely throw away RELISTOR pre-filled syringes and attached needle after use.
- To avoid needle-stick injuries, do not recap used needles.
- Avoid touching the trigger fingers of the RELISTOR pre-filled syringe to keep from activating the needle guard (safety device) too soon. The needle guard is activated by pressure from the plunger on the trigger fingers (See Figure A).
Gather the supplies you will need for your injection (See Figure A). These include:
- 1 RELISTOR pre-filled syringe with attached needle
- 1 alcohol swab
- 1 cotton ball or gauze
- 1 adhesive bandage
- a container to dispose of used pre-filled syringesandneedles. See Step 4: “Dispose of used pre-filled syringes and needles.”
Step 1: Choose and prepare the injection site
- Choose an injection site on your abdomen, thighs, or upper arms. See the shaded areas in Figures B and C below. Do not inject at the exact same spot each time (rotate injection sites). Do not inject into areas where the skin is tender, bruised, red or hard. Avoid areas with scars or stretch marks.
Figure B Abdomen or thigh – use these sites when injecting yourself or another person.
Figure C Upper arm – use this site only when injecting another person.
- Clean the injection site with an alcohol swab and let it air dry. Do not touch this area again before giving the injection (See Figure D).
Step 2: Prepare the pre-filled syringe
- Choose a flat, clean, well-lit work surface.
- Wash your hands with soap and water before preparing for the injection.
- Look at the pre-filled syringe of RELISTOR (See Figure E). Make sure that the dose prescribed by your healthcare provider matches the dose on the pre-filled syringe label. Look at the plunger rod of the syringe. If the dose prescribed by your healthcare provider is 8 mg, the plunger rod will be yellow; if the prescribed dose is 12 mg, the plunger rod of the syringe will be dark blue (See Figure E).
- The liquid in the pre-filled syringe should be clear and colorless to pale yellow, and should not have any particles in it. Do not use the pre-filled syringe if it looks discolored, cloudy, or has any particles.
- Use one hand to firmly hold the barrel of the pre-filled syringe. Use your other hand to pull the needle cap straight off (Figure F). Do not touch the needle or allow it to touch anything.
Step 3: Inject RELISTOR
- Use one hand to pinch the skin around the injection site (See Figure G).
- Use your other hand to hold the pre-filled syringe. Insert the full length of the needle into the skin at a 45-degree angle with a quick “dart‑like” motion (See Figure H).
- Let go of the skin and slowly push the plunger in with your thumb until the pre-filled syringe is empty (See Figure I). This will release the needle guard (safety device).
- Continue to hold pressure on the plunger with your thumb and quickly pull the needle out of the skin. Be careful to keep the needle at the same angle as it was inserted. Remove your thumb from the plunger to allow the protective sleeve to cover the needle (See Figure J). There may be a little bleeding at the injection site.
- Hold a cotton ball or gauze over the injection site (See Figure K). Do not rub the injection site. Apply an adhesive bandage to the injection site if needed.
Step 4: Dispose of used pre-filled syringes and needles
- Do not re-use the pre-filled syringeand attached needle.
- To avoid needle-stick injuries, do not recapused needles.
- Put yourused pre-filledsyringes and attached needlesin a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistantlid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside thecontainer.
- When your sharps disposalcontainer is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal .
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- If you have any questions, talk to your healthcare provider or pharmacist.
How should I store RELISTOR?
- Store pre-filled syringes at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze RELISTOR.
- Keep RELISTOR away from light until you are ready to use it.
Keep RELISTOR and all medicines, needles and syringes out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:

Salix Pharmaceuticals, Inc.
Raleigh, NC 27615
Under license from:

Progenics Pharmaceuticals, Inc.
Tarrytown, NY 10591
Revised AUG 2013
Product protected by U.S. Patent Nos. 6,559,158, 8,247,425, and 8,420,663.
See www.salix.com for patent information
Patient Instructions for Use of RELISTORVIAL AND SYRINGE WITH RETRACTABLE NEEDLE IN TRAY
Instructions for Use
RELISTOR® (rel-i-store)
(methylnaltrexone bromide)
Vial and Syringe with Retractable Needle in Tray
Read this Instructions for Use before you start using RELISTOR and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
The following instructions explain how to prepare and give an injection of RELISTOR the right way, when using a RELISTOR tray containing a syringe with a retractable needle. A retractable needle is one that is pulled back so that it is covered after use, to prevent needle-stick injury.
Important information:
- Do not use a RELISTOR vial more than one time, even if there is medicine left in the vial.
- If RELISTOR has been drawn into a syringe and you are unable to use the medicine right away, carefully recap the needle and keep the syringe at room temperature for up to 24 hours. The syringe does not need to be kept away from light during the 24-hour period. For more information about how to store RELISTOR, see the section called “How should I store RELISTOR?” at the end of this Instructions for Use.
- Safely throw away RELISTOR vials after use.
- Do not reuse syringes and needles. See Step 5: “Dispose of used syringes and needles” for information about how to safely throw away used needles and syringes.
- To avoid needle-stick injuries, do not recap used needles.
Your tray should include (See Figure A):
- 1 RELISTOR vial
- 1 1 mL syringe with retractable needle (VanishPoint®)
- 2 alcohol swabs
You will also need:
- 1 cotton ball or gauze
- 1 adhesive bandage
- a container to dispose of your used syringes andneedles. See Step 5: “Dispose of used syringes and needles.”
Vial and Syringe Parts
Step 1: Choose and prepare the injection site
- Choose an injection site on your abdomen, thighs, or upper arms. See the shaded areas in B and C below. Do not inject at the exact same spot each time (rotate injection sites). Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas with scars or stretch marks.
Figure B Abdomen or thigh – use these sites when injecting yourself or another person.
Figure C Upper arm – use this site only when injecting another person.
- Clean the injection site with an alcohol swab and let it air dry. Do not touch this area again before giving the injection (See Figure D).
Step 2: Prepare the injection
- Choose a flat, clean, well-lit work surface.
- Wash your hands with soap and water before preparing for the injection.
- Look at the vial of RELISTOR (See Figure E). The liquid in the vial should be clear and colorless to pale yellow, and should not have any particles in it. Do not use the vial if it looks discolored, cloudy, or has any particles.
Step 3: Prepare the syringe
- Remove the cap from the vial containing RELISTOR (See Figure F).
- Wipe the rubber stopper with an alcohol swab (See Figure G).
- Firmly hold the barrel of the syringe with one hand. With your other hand, pull the needle cap straight off (See Figure H). Do not touch the needle or allow it to touch anything
- Carefully pull back on the plunger to the line that matches the dose prescribed by your healthcare provider (See Figures I and J). For most people, this will be the 0.4 mL mark which is an 8 mg dose or the 0.6 mL mark which is a 12 mg dose.
- Use one hand to hold the vial steady. Use your other hand to insert the needle straight down into the rubber top of the RELISTOR vial (See Figure K). Do not insert it at an angle. This may cause the needle to bend or break. You will feel some resistance as the needle passes through the rubber top.
- Gently push down on the plunger until you feel resistance, and most of the air has gone from the syringe into the vial (See Figure L). Stop pushing down on the plunger when you feel resistance. If you continue to push down on the plunger when you feel resistance, the needle will pull back (retract) into the syringe barrel.
- With the needle still in the vial, turn the vial and syringe upside down. Hold the syringe at eye level. Make sure the tip of the needle is in the fluid. Slowly pull back on the plunger (See Figure M) until the tip lines up with the mark that matches your prescribed dose. For most people, this will be the 0.4 mL mark which is an 8 mg dose or the 0.6 mL mark which is a 12 mg dose.
- You may see some fluid or bubbles inside the vial when the syringe is filled. This is normal.
- With the needle still in the vial, gently tap the syringe to make any air bubbles rise to the top (See Figure N).
- Gently push the plunger up until all air bubbles are out of the syringe (See Figure O). A small air bubble may stay in the syringe. This is okay and it will not affect the dose of medicine in the syringe.
- Make sure the tip of the needle is in the fluid. Slowly pull back the plunger to draw the right amount of liquid back into the syringe (See Figure P).
Check to be sure that you have the right dose of RELISTOR in the syringe.
- Slowly withdraw the needle from the vial. Do not touch the needle or allow it to touch anything). Safely throw away the vial with any unused medicine.
Step 4: Inject RELISTOR
- Use one hand to pinch the skin around the injection site (See Figure Q).
- Use your other hand to hold the syringe. Insert the full length of the needle into the skin at a 45-degree angle with a “quick dart-like” motion (See Figure R).
- Let go of the skin and slowly push in on the plunger past the resistance point, until the syringe is empty and you hear a click (See Figure S).
- The click sound means that the needle (T) has been pulled back (retracted) into the syringe barrel (See Figure U). You can now remove the syringe from your skin.
- Hold a cotton ball or gauze over the injection site (See Figure V). Do not rub the injection site. Apply an adhesive bandage to the injection site if needed.
Step 5: Dispose of used syringes and needles
- Do not re-use syringes or needles.
- To avoid needle-stick injuries, do not recap used needles.
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- If you have any questions, talk to your healthcare provider or pharmacist.
How should I store RELISTOR?
- Store RELISTOR vials at room temperature between 68°F to 77°F (20 °C to 25°C).
- Do not freeze RELISTOR.
- Keep RELISTOR away from light until you are ready to use it.
- If RELISTOR has been drawn into a syringe and you are unable to use the medicine right away, keep the syringe at room temperature for up to 24 hours. The syringe does not need to be kept away from light during the 24-hour period.
Keep RELISTOR and all medicines, needles and syringes out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:

Salix Pharmaceuticals, Inc.
Raleigh, NC 27615
Under license from:

Progenics Pharmaceuticals, Inc.
Tarrytown, NY 10591
Revised AUG 2013
Product protected by U.S. Patent Nos. 6,559,158, 8,247,425, and 8,420,663.
See www.salix.com for patent information
Patient Instructions for Use of RELISTOR® VIAL AND STANDARD SYRINGE AND NEEDLE
Instructions for Use
RELISTOR® (rel-i-store)
(methylnaltrexone bromide)
Subcutaneous Injection
Vial
Read this Instructions for Use before you start using RELISTOR and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
The following instructions explain how to prepare and give an injection of RELISTOR the right way, when using a vial of RELISTOR.
Important information:
- Use the syringes and needles prescribed by your healthcare provider.
- Do not use a RELISTOR vial more than one time, even if there is medicine left in the vial.
- If RELISTOR has been drawn into a syringe and you are unable to use the medicine right away, carefully recap the needle and keep the syringe at room temperature for up to 24 hours. The syringe does not need to be kept away from light during the 24-hour period. For more information about how to store RELISTOR, see the section “ How should I store RELISTOR?” at the end of this Instructions for Use.
- Safely throw away RELISTOR vials after use.
- Do not re-use syringes or needles. See Step 5 “Dispose of used syringes and needles” for information about how to safely throw away used needles and syringes.
- To avoid needle-stick injuries, do not recap used needles.
Gather the supplies you will need for your injection (See Figure A.). These include:
- 1 RELISTOR vial
- 1 1 mL syringe with a 27-gauge, ½ inch needle for subcutaneous use
- 2 alcohol swabs
- 1 cotton ball or gauze
- 1 adhesive bandage
- a container to dispose of used syringesandneedles. See Step 5: “Dispose of used syringes and needles.”
Vial and Syringe Parts
Step 1: Choose and prepare the injection site
- Choose an injection site on your abdomen, thighs, or upper arms. See the shaded areas in Figures B and C below. Do not inject at the exact same spot each time (rotate injection sites). Do not inject into areas where the skin is tender, bruised, red or hard. Avoid areas with scars or stretch marks.
Figure B Abdomen or thigh – use these sites when injecting yourself or another person.
Figure C Upper arm – use this site only when injecting another person.
- Clean the injection site with an alcohol swab and let it air dry. Do not touch this area again before giving the injection (See Figure D).
Step 2: Prepare the injection
- Choose a flat, clean, well-lit work surface.
- Wash your hands with soap and water before preparing for the injection.
- Look at the vial of RELISTOR (See Figure E). The liquid in the vial should be clear and colorless to pale yellow, and should not have any particles in it. Do not use the vial if it looks discolored, cloudy, or has any particles.
Step 3: Prepare the syringe
- Remove the cap from the RELISTOR vial (See Figure F).
- Wipe the rubber stopper with an alcohol swab (See Figure G).
- Firmly hold the barrel of the syringe with one hand. With your other hand, pull the needle cap straight off (See Figure H). Do not touch the needle or allow it to touch anything.
- Carefully pull back on the plunger to the line that matches the dose prescribed by your healthcare provider (See Figures I and J). For most people, this will be the 0.4 mL mark which is an 8 mg dose or the 0.6 mL mark which is a 12 mg dose.
- Use one hand to hold the vial steady. Use your other hand to insert the needle straight down into the rubber top of the vial (See Figure K). Do not insert it at an angle. This may cause the needle to bend or break. You will feel some resistance as the needle passes through the rubber top.
- Gently push down the plunger until all of the air has gone from the syringe into the vial (See Figure L).
- With the needle still in the vial, turn the vial and syringe upside down. Hold the syringe at eye level. Make sure the tip of the needle is in the fluid. Slowly pull back on the plunger (See Figure M) until the tip lines up with the mark that matches your prescribed dose. For most people, this will be the 0.4 mL mark which is an 8 mg dose or the 0.6 mL mark which is a 12 mg dose.
- You may see some fluid or bubbles inside the vial when the syringe is filled. This is normal.
- With the needle still in the vial, gently tap the side of the syringe to make any air bubbles rise to the top (See Figure N).
- Slowly push the plunger up until all air bubbles are out of the syringe (See Figure O). A small air bubble may stay in the syringe. This is okay and it will not affect the dose of medicine in the syringe.
- Make sure the tip of the needle is in the fluid. Slowly pull back the plunger to draw the right amount of liquid back into the syringe (See Figure P).
Check to be sure that you have the right dose of RELISTOR in the syringe.
- Slowly withdraw the needle from the vial. Do not touch the needle or allow it to touch anything. Safely throw away the vial with any unused medicine.
Step 4: Inject RELISTOR
- Use one hand to pinch the skin around the injection site (See Figure Q).
- Use your other hand to hold the syringe. Insert the full length of the needle into the skin at a 45-degree angle with a quick “dart‑like” motion (See Figure R).
- Let go of the skin and slowly push in on the plunger until the syringe is empty (Figure S).
- When the syringe is empty, quickly pull the needle out of the skin, being careful to keep it at the same angle as it was inserted. There may be a little bleeding at the injection site.
- Hold a cotton ball or gauze over the injection site (Figure T). Do not rub the injection site. Apply an adhesive bandage to the injection site if needed.
Step 5: Dispose of used syringes and needles
- Do not re-use a syringe or needle.
- To avoid needle-stick injuries, do not recap a used needle.
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- If you have any questions, talk to your healthcare provider or pharmacist.
How should I store RELISTOR?
- Store RELISTOR vials at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze RELISTOR.
- Keep RELISTOR away from light until you are ready to use it.
- If RELISTOR has been drawn into a syringe and you are unable to use the medicine right away, keep the syringe at room temperature for up to 24 hours. The syringe does not need to be kept away from light during the 24-hour period.
Keep RELISTOR and all medicines, needles and syringes out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:

Salix Pharmaceuticals, Inc.
Raleigh, NC 27615
Under license from:

Progenics Pharmaceuticals, Inc.
Tarrytown, NY 10591
Revised AUG 2013
Product protected by U.S. Patent Nos. 6,559,158, 8,247,425, and 8,420,663.
See www.salix.com for patent information
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - VIAL
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per vial
For Subcutaneous Injection Only
Sterile Single Use Vial
Discard After Use
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON
NDC 65649-551-02
RELISTOR™
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6ml per vial
For Subcutaneous Injection Only
1 Sterile Single Use Vial
Discard After Use
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - TRAY
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6ml per vial
For Subcutaneous Injection Only
Contains one sterile single use vial
Discard After Use
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON
NDC 65649-553-05
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6ml per vial
For Subcutaneous Injection Only
Each carton contains 7 trays. Each tray contains one sterile single use vial.
Discard after use.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 8 mg/0.4 mL - SYRINGE LABEL
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
8 mg/0.4 mL per syringe
For Subcutaneous Injection Only
Sterile Single Use
Discard After use
Protect from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 8 mg/0.4 mL - SYRINGE LIDDING
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
8 mg/0.4 mL per syringe
For Subcutaneous Injection Only
Contains 1 Pre-filled Syringe with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 8 mg/0.4 mL - CARTON
NDC 65649-552-04
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
8 mg/0.4 mL per syringe
For Subcutaneous Injection Only
Contains 7 Pre-filled Syringes with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LABEL
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Sterile Single Use
Discard After use
Protect from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LIDDING
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Contains 1 Pre-filled Syringe with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON
NDC 65649-551-03
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Contains 7 Pre-filled Syringes with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LABEL - SINGLE
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Sterile Single Use
Discard After use
Protect from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LIDDING - SINGLE
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Contains 1 Pre-filled Syringe with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON - SINGLE
NDC 65649-551-07
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Contains 1 Pre-filled Syringes with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LABEL - SINGLE - SAMPLE
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Sterile Single Use
Discard After use
Protect from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - SYRINGE LIDDING - SINGLE - SAMPLE
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Contains 1 Pre-filled Syringe with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 12 mg/0.6 mL - CARTON - SINGLE - SAMPLE
NDC 65649-551-06
RELISTOR®
(methylnaltrexone bromide)
Subcutaneous Injection
12 mg/0.6 mL per syringe
For Subcutaneous Injection Only
Contains 1 Pre-filled Syringes with Needle Guard
Single Use Only. Discard after use.
Protect syringe from light.
Rx only
Manufactured for Salix Pharmaceuticals, Inc.
Under license from Progenics Pharmaceuticals
RelistorMethylnaltrexone bromide KIT
| |||||||||||||||||||||||||||||||||||||||||||||
RelistorMethylnaltrexone bromide INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
RelistorMethylnaltrexone bromide INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||