Revalife
International Nutraceutical Company of America (INCA), LLC
International Nutraceutical Company of Americe (INCA), LLC
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient : Menthol 3%
Purpose
Purpose-Topical Analgesic
Uses
Uses-Temporarily relieves minor pain of joints and muscles associated with arthritis, bursitis, tendonitis, strains
Directions: Apply to clean, dry skin over painful joint or muscle gently massaging until cream disappears. For optimum results use daily for 30 days and continue to use daily thereafter. Repeat as neccessary. Close cap tightly after use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
For external use only. Do not bandage tightly or use with a heating pad. Avoid contact with mouth, eyes, and mucous membranes. Do not apply to wounds, damaged, broken, or irritated skin.
Stop use and ask a doctor if: Symptoms persists over 7 day or clear up and occur again within a few days. Redness is present or irritation develops. Using on a child 12 years of age or under.
Inactive Ingredients: EDTA, Ethanol, Isopropyl palmitate, Methyl Paraben, Monosodium Phosphate, N-Acetyl Glucosamine, Poloxamer 407, Propyl Paraben, Soy Lecithin, Todopherol Acetate (Vitamin E), Water
MM1
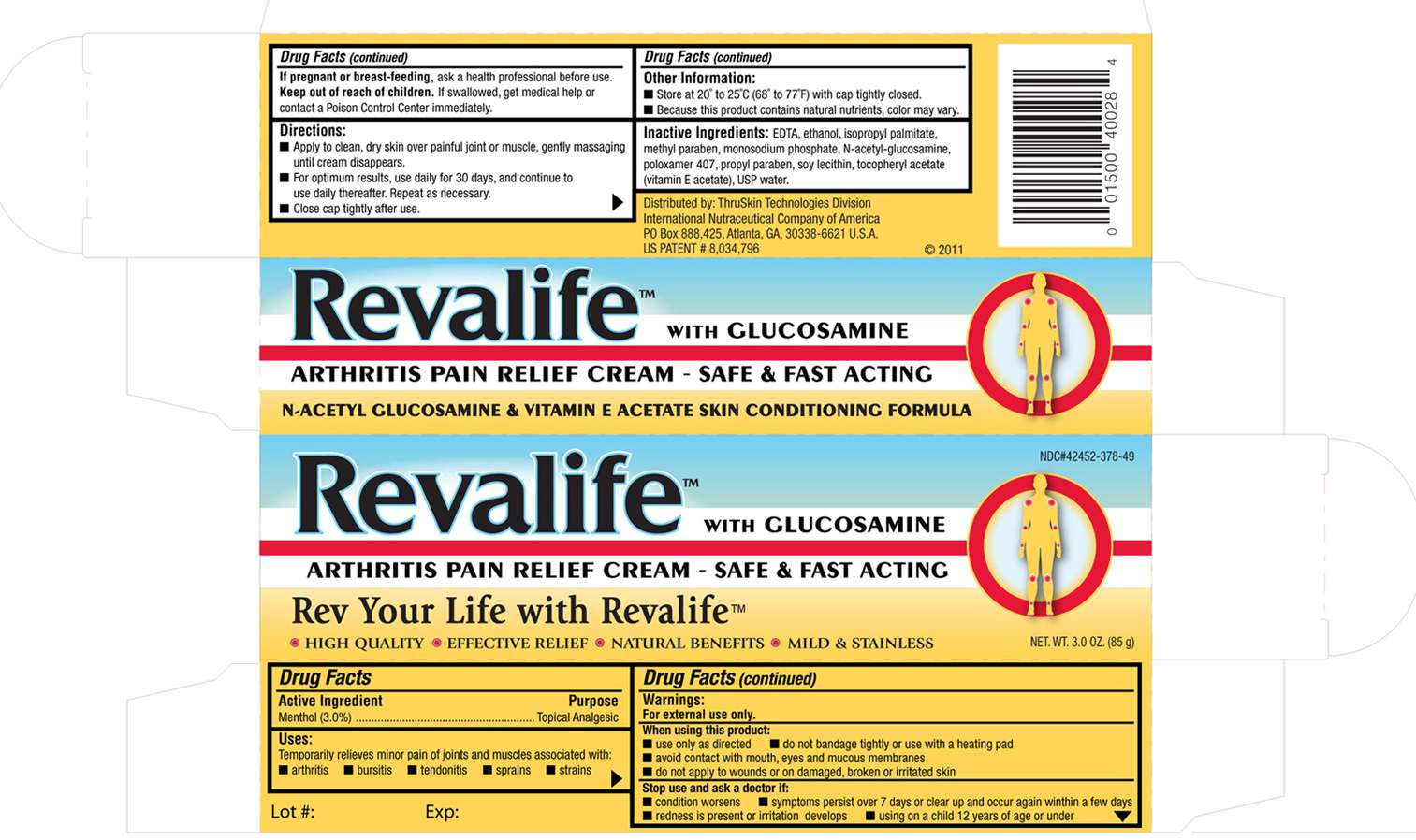
If pregnant or breast feeding, ask a health professional before use
RevalifeTopical menthol OINTMENT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||