Rimmel London
Rimmel London Moisture Renew Lipgloss Mauve Renew (125) SPF 15 Sunscreen
FULL PRESCRIBING INFORMATION
Uses
Active ingredient
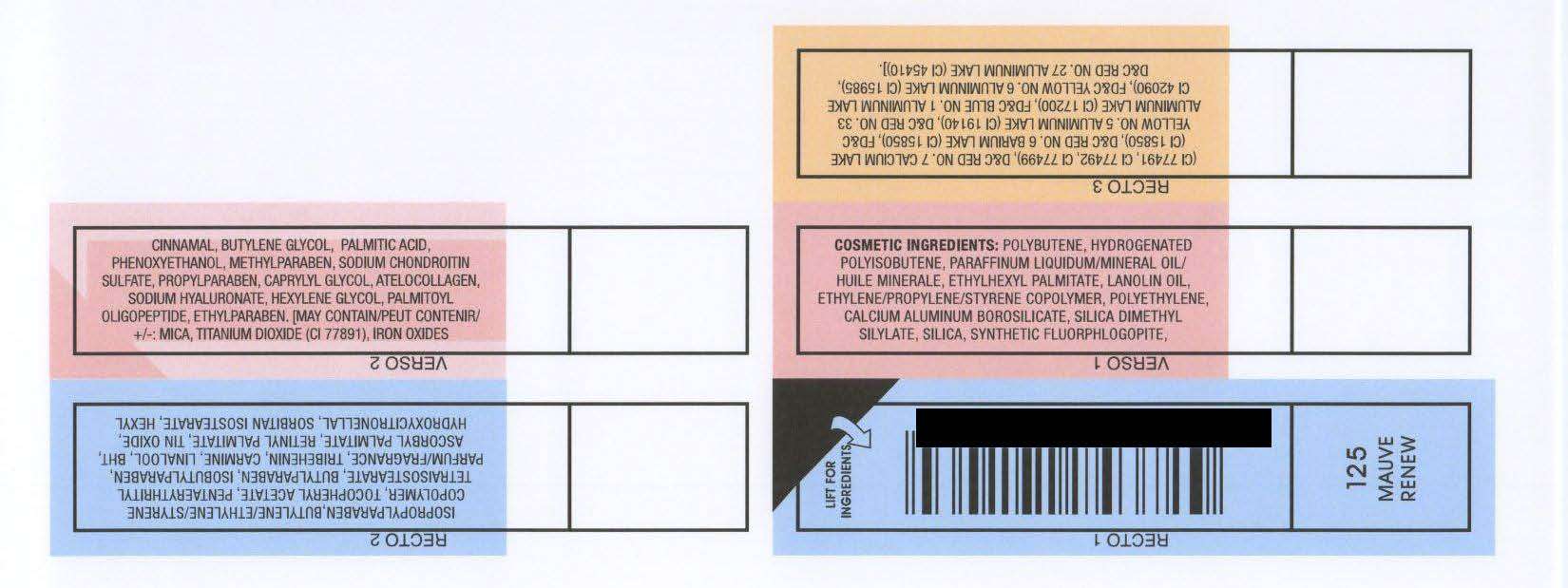
COSMETIC INGREDIENTS: POLYBUTENE, HYDROGENATED
POLYISOBUTENE, PARAFFINUM LIQUIDUM/MINERAL OIL/
HUILE MINERALE, ETHYLHEXYL PALMITATE, LANOLIN OIL,
ETHYLENE/PROPYLENE/STYRENE COPOLYMER, POLYETHYLENE,
CALCIUM ALUMINUM BOROSILICATE, SILICA DIMETHYL
SILYLATE, SILICA, SYNTHETIC FLUORPHLOGOPITE,
ISOPROPYLPARABEN,BUTYLENE/ETHYLENE/STYRENE
COPOLYMER, TOCOPHERYL ACETATE, PENTAERYTHRITYL
TETRAISOSTEARATE, BUTYLPARABEN, ISOBUTYLPARABEN,
PARFUM/FRAGRANCE, TRIBEHENIN, CARMINE, LINALOOL, BHT,
ASCORBYL PALMITATE, RETINYL PALMITATE, TIN OXIDE,
HYDROXYCITRONELLAL, SORBITAN ISOSTEARATE, HEXYL
CINNAMAL, BUTYLENE GLYCOL, PALMITIC ACID,
PHENOXYETHANOL, METHYLPARABEN, SODIUM CHONDROITIN
SULFATE, PROPYLPARABEN, CAPRYLYL GLYCOL, ATELOCOLLAGEN,
SODIUM HYALURONATE, HEXYLENE GLYCOL, PALMITOYL
OLIGOPEPTIDE, ETHYLPARABEN. [MAY CONTAIN/PEUT CONTENIR/
: MICA, TITANIUM DIOXIDE (CI 77891), IRON OXIDES
(CI 77491, CI 77492, CI 77499), DandC RED NO. 7 CALCIUM LAKE
(CI 15850), DandC RED NO. 6 BARIUM LAKE (CI 15850), FDandC
YELLOW NO. 5 ALUMINUM LAKE (CI 19140), DandC RED NO. 33
ALUMINUM LAKE (CI 17200), FDandC BLUE NO. 1 ALUMINUM LAKE
CI 42090), FDandC YELLOW NO. 6 ALUMINUM LAKE (CI 15985),
DandC RED NO. 27 ALUMINUM LAKE (CI 45410)].
RIMMEL LONDON
SPF 15 SUNSCREEN
MOISTURE RENEW
WITH COLLAGEN AND VITAMINS A, C and E
CREAM LIPGLOSS
0.20 fl oz 6 ml

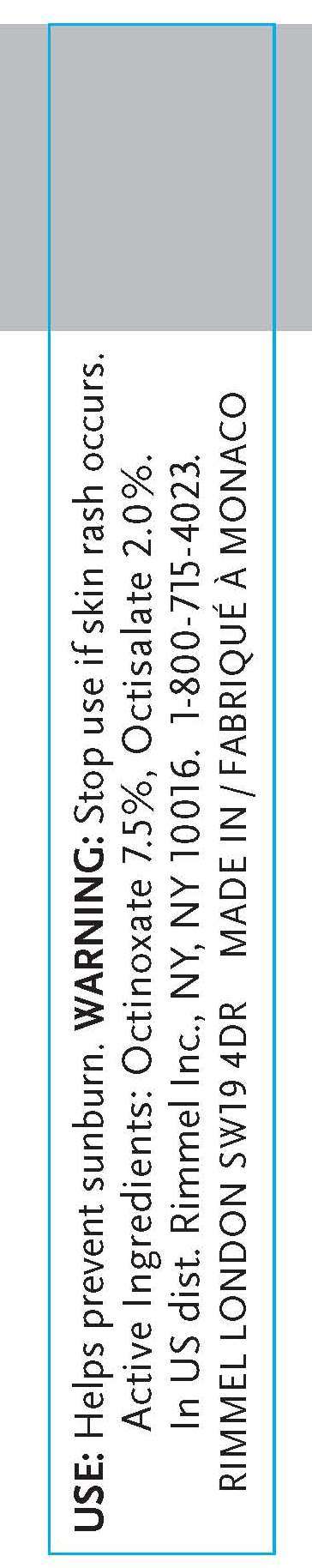
Rimmel LondonOctinoxate, Octisalate CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||