ropinirole
Dispensing Solutions, Inc.
PSS World Medical, Inc.
rOPINIRole Tablets USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ROPINIROLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ROPINIROLE INDICATIONS AND USAGE
- ROPINIROLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ROPINIROLE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- ROPINIROLE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PATIENT INFORMATION - Restless Legs Syndrome
- What are ropinirole tablets?
- What is the most important information I should know about ropinirole tablets?
- Who should not take ropinirole tablets?
- What should I tell my doctor?
- How should I take ropinirole tablets for RLS?
- What are the possible side effects of ropinirole tablets?
- Other Information about ropinirole tablets
- PATIENT INFORMATION - Parkinson's Disease
- What are ropinirole tablets?
- What is the most important information I should know about ropinirole tablets?
- Who should not take ropinirole tablets?
- What should I tell my doctor?
- How should I take ropinirole tablets for Parkinson's disease?
- What are the possible side effects of ropinirole tablets?
- Other Information about ropinirole tablets
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Patient Information Included
ROPINIROLE DESCRIPTION
Ropinirole hydrochloride USP is an orally administered non-ergoline dopamine agonist. It is the hydrochloride salt of 4-[2-(dipropylamino) ethyl]-1,3-dihydro-2H-indol-2-one monohydrochloride and has an empirical formula of C16H24N2O•HCl. The molecular weight is 296.84 (260.38 as the free base).
The structural formula is:
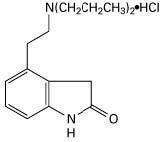
Ropinirole hydrochloride USP is a pale cream to yellow powder with a melting range of 243° to 250°C and a solubility of 133 mg/mL in water.
Each circular film-coated tablet with beveled edges contains ropinirole hydrochloride USP equivalent to ropinirole 2 mg. Inactive ingredients consist of: citric acid anhydrous powder, croscarmellose sodium, lactose anhydrous, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and one or more of the following: carmine, aluminum lake, hypromellose, ferric oxide red, polyethylene glycol, talc and titanium dioxide.
The product meets USP Dissolution test 2.
CLINICAL PHARMACOLOGY
Mechanism of Action:
Ropinirole hydrochloride is a non-ergoline dopamine agonist with high relative in vitro specificity and full intrinsic activity at the D2 and D3 dopamine receptor subtypes, binding with higher affinity to D3 than to D2 or D4 receptor subtypes.
Ropinirole has moderate in vitro affinity for opioid receptors. Ropinirole and its metabolites have negligible in vitro affinity for dopamine D1, 5-HT1, 5-HT2, benzodiazepine, GABA, muscarinic, alpha1-, alpha2-, and beta-adrenoreceptors.
Parkinson’s Disease:
The precise mechanism of action of ropinirole hydrochloride as a treatment for Parkinson’s disease is unknown, although it is believed to be due to stimulation of postsynaptic dopamine D2-type receptors within the caudate-putamen in the brain. This conclusion is supported by studies that show that ropinirole improves motor function in various animal models of Parkinson’s disease. In particular, ropinirole attenuates the motor deficits induced by lesioning the ascending nigrostriatal dopaminergic pathway with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in primates. The relevance of D3 receptor binding in Parkinson’s disease is unknown.
Restless Legs Syndrome (RLS):
The precise mechanism of action of ropinirole hydrochloride as a treatment for Restless Legs Syndrome (also known as Ekbom Syndrome) is unknown. Although the pathophysiology of RLS is largely unknown, neuropharmacological evidence suggests primary dopaminergic system involvement. Positron emission tomographic (PET) studies suggest that a mild striatal presynaptic dopaminergic dysfunction may be involved in the pathogenesis of RLS.
Clinical Pharmacology Studies:
In healthy normotensive subjects, single oral doses of ropinirole hydrochloride in the range 0.01 to 2.5 mg had little or no effect on supine blood pressure and pulse rates. Upon standing, ropinirole hydrochloride caused decreases in systolic and diastolic blood pressure at doses above 0.25 mg. In some subjects, these changes were associated with the emergence of orthostatic symptoms, bradycardia, and, in one case, transient sinus arrest with syncope. With repeat dosing and slow titration up to 4 mg once daily in healthy volunteers, postural hypotension or hypotension-related adverse events were noted in 13% of subjects on ropinirole hydrochloride and none of the subjects on placebo.
The mechanism of postural hypotension induced by ropinirole hydrochloride is presumed to be due to a D2-mediated blunting of the noradrenergic response to standing and subsequent decrease in peripheral vascular resistance. Nausea is a common concomitant symptom of orthostatic signs and symptoms.
At oral doses as low as 0.2 mg, ropinirole hydrochloride suppressed serum prolactin concentrations in healthy male volunteers.
Ropinirole hydrochloride had no dose-related effect on ECG wave form and rhythm in young, healthy, male volunteers in the range of 0.01 to 2.5 mg.
Ropinirole hydrochloride had no dose-or exposure-related effect on mean QT intervals in healthy male and female volunteers titrated to doses up to 4 mg/day. The effect of ropinirole hydrochloride on QT intervals at higher exposures achieved either due to drug interactions or at doses used in Parkinson’s disease has not been systematically evaluated.
Pharmacokinetics:
Absorption, Distribution, Metabolism, and Elimination:
The pharmacokinetics of ropinirole are similar in Parkinson's disease patients and patients with Restless Legs Syndrome. Ropinirole is rapidly absorbed after oral administration, reaching peak concentration in approximately 1-2 hours. In clinical studies, over 88% of a radiolabeled dose was recovered in urine and the absolute bioavailability was 55%, indicating a first-pass effect. Relative bioavailability from a tablet compared to an oral solution is 85%. Food does not affect the extent of absorption of ropinirole, although its Tmax is increased by 2.5 hours and its Cmax is decreased by approximately 25% when the drug is taken with a high-fat meal. The clearance of ropinirole after oral administration to patients is 47 L/hr (cv = 45%) and its elimination half-life is approximately 6 hours. Ropinirole is extensively metabolized by the liver to inactive metabolites and displays linear kinetics over the therapeutic dosing range of 1 to 8 mg 3 times daily. Steady-state concentrations are expected to be achieved within 2 days of dosing. Accumulation upon multiple dosing is predictive from single dosing.
Ropinirole is widely distributed throughout the body, with an apparent volume of distribution of 7.5 L/kg (cv = 32%). It is up to 40% bound to plasma proteins and has a blood-to-plasma ratio of 1:1.
The major metabolic pathways are N-despropylation and hydroxylation to form the inactive N-despropyl and hydroxy metabolites. In vitro studies indicate that the major cytochrome P450 isozyme involved in the metabolism of ropinirole is CYP1A2, an enzyme known to be stimulated by smoking and omeprazole, and inhibited by, for example, fluvoxamine, mexiletine, and the older fluoroquinolones such as ciprofloxacin and norfloxacin. The N-despropyl metabolite is converted to carbamyl glucuronide, carboxylic acid, and N-despropyl hydroxy metabolites. The hydroxy metabolite of ropinirole is rapidly glucuronidated. Less than 10% of the administered dose is excreted as unchanged drug in urine. N-despropyl ropinirole is the predominant metabolite found in urine (40%), followed by the carboxylic acid metabolite (10%), and the glucuronide of the hydroxy metabolite (10%).
P Interaction:
In vitro metabolism studies showed that CYP1A2 was the major enzyme responsible for the metabolism of ropinirole. Inhibitors or inducers of this enzyme have been shown to alter its clearance when coadministered with ropinirole. Therefore, if therapy with a drug known to be a potent inhibitor of CYP1A2 is stopped or started during treatment with ropinirole hydrochloride, adjustment of the dose of ropinirole hydrochloride may be required.
Population Subgroups:
Because therapy with ropinirole hydrochloride is initiated at a low dose and gradually titrated upward according to clinical tolerability to obtain the optimum therapeutic effect, adjustment of the initial dose based on gender, weight, or age is not necessary.
Age:
Oral clearance of ropinirole is reduced by 30% in patients above 65 years of age compared to younger patients. Dosage adjustment is not necessary in the elderly (above 65 years), as the dose of ropinirole is to be individually titrated to clinical response.
Gender:
Female and male patients showed similar oral clearance.
Race:
The influence of race on the pharmacokinetics of ropinirole has not been evaluated.
Cigarette Smoking:
Smoking is expected to increase the clearance of ropinirole since CYP1A2 is known to be induced by smoking. In a study in patients with RLS, smokers (n = 7) had an approximate 30% lower Cmax and a 38% lower AUC than did nonsmokers (n = 11), when those parameters were normalized for dose.
Renal Impairment:
Based on population pharmacokinetic analysis, no difference was observed in the pharmacokinetics of ropinirole in patients with moderate renal impairment (creatinine clearance between 30 to 50 mL/min.) compared to an age-matched population with creatinine clearance above 50 mL/min. Therefore, no dosage adjustment is necessary in moderately renally impaired patients. The use of ropinirole hydrochloride in patients with severe renal impairment has not been studied.
The effect of hemodialysis on drug removal is not known, but because of the relatively high apparent volume of distribution of ropinirole (525 L), the removal of the drug by hemodialysis is unlikely.
Hepatic Impairment:
The pharmacokinetics of ropinirole have not been studied in hepatically impaired patients. These patients may have higher plasma levels and lower clearance of the drug than patients with normal hepatic function. The drug should be titrated with caution in this population.
Other Diseases:
Population pharmacokinetic analysis revealed no change in the oral clearance of ropinirole in patients with concomitant diseases such as hypertension, depression, osteoporosis/arthritis, and insomnia compared to patients with Parkinson’s disease only.
Clinical Trials:
Parkinson's Disease:
The effectiveness of ropinirole hydrochloride in the treatment of Parkinson’s disease was evaluated in a multinational drug development program consisting of 11 randomized, controlled trials. Four were conducted in patients with early Parkinson’s disease and no concomitant levodopa (L-dopa), and 7 were conducted in patients with advanced Parkinson’s disease with concomitant L-dopa.
Among these 11 studies, 3 placebo-controlled studies provide the most persuasive evidence of ropinirole’s effectiveness in the management of patients with Parkinson’s disease who were and were not receiving concomitant L-dopa. Two of these 3 trials enrolled patients with early Parkinson’s disease (without L-dopa) and 1 enrolled patients receiving L-dopa.
In these studies a variety of measures were used to assess the effects of treatment (e.g., the Unified Parkinson’s Disease Rating Scale [UPDRS], Clinical Global Impression [CGI] scores, patient diaries recording time “on” and “off,” and tolerability of L-dopa dose reductions).
In both studies of early Parkinson’s disease (without L-dopa) patients, the motor component (Part III) of the UPDRS was the primary outcome assessment. The UPDRS is a 4-part multi-item rating scale intended to evaluate mentation (Part I), activities of daily living (Part II), motor performance (Part III), and complications of therapy (Part IV). Part III of the UPDRS contains 14 items designed to assess the severity of the cardinal motor findings in patients with Parkinson’s disease (e.g., tremor, rigidity, bradykinesia, postural instability, etc.) scored for different body regions and has a maximum (worst) score of 108. Responders were defined as patients with at least a 30% reduction in the Part III score.
In the study of advanced Parkinson’s disease (with L-dopa) patients, both reduction in percent awake time spent “off” and the ability to reduce the daily use of L-dopa were assessed as a combined endpoint and individually.
Studies in Patients With Early Parkinson’s Disease (Without L-dopa):
One early therapy study was a 12-week multicenter study in which 63 patients (41 on ropinirole hydrochloride) with idiopathic Parkinson’s disease receiving concomitant anti-Parkinson medication (but not L-dopa) were randomized to either ropinirole hydrochloride or placebo. Patients had a mean disease duration of approximately 2 years. Patients were eligible for enrollment if they presented with bradykinesia and at least tremor, rigidity, or postural instability. In addition, they must have been classified as Hoehn & Yahr Stage I-IV. This scale, ranging from I = unilateral involvement with minimal impairment to V = confined to wheelchair or bed, is a standard instrument used for staging patients with Parkinson’s disease. The primary outcome measure in this trial was the proportion of patients experiencing a decrease (compared to baseline) of at least 30% in the UPDRS motor score.
Patients were titrated for up to 10 weeks, starting at 0.5 mg twice daily, with weekly increments of 0.5 mg twice daily to a maximum of 5 mg twice daily. Once patients reached their maximally tolerated dose (or 5 mg twice daily), they were maintained on that dose through 12 weeks. The mean dose achieved by patients at study endpoint was 7.4 mg/day. At the end of 12 weeks, 71% of patients treated with ropinirole hydrochloride were responders, compared with 41% of patients in the placebo group (p = 0.021).
Statistically significant differences between the percentage of responders on ropinirole hydrochloride compared to placebo were seen after 8 weeks of treatment.
In addition, the mean percentage improvement from baseline in the Total Motor Score was 43% in patients treated with ropinirole hydrochloride compared with 21% in patients treated with placebo (p = 0.018).
Statistically significant differences in UPDRS motor score between ropinirole hydrochloride and placebo were seen after 2 weeks of treatment.
The median daily dose at which a 30% reduction in UPDRS motor score was sustained was 4 mg.
The second trial in early Parkinson’s disease (without L-dopa) patients was a double-blind, randomized, placebo-controlled, 6-month study. Patients were essentially similar to those in the study described above; concomitant use of selegiline was allowed, but patients were not permitted to use anticholinergics or amantadine during the study. Patients had a mean disease duration of 2 years and limited (not more than a 6-week period) or no prior exposure to L-dopa. The starting dose of ropinirole hydrochloride in this trial was 0.25 mg 3 times daily. The dose was titrated at weekly intervals by increments of 0.25 mg 3 times daily to a dose of 1 mg 3 times daily. Further titrations at weekly intervals were at increments of 0.5 mg 3 times daily up to a dose of 3 mg 3 times daily, and then weekly at increments of 1 mg 3 times daily. Patients were to be titrated to a dose of at least 1.5 mg 3 times daily and then to their maximally tolerated dose, up to a maximum of 8 mg 3 times daily. The mean dose attained in patients at study endpoint was 15.7 mg/day.
The primary measure of effectiveness was the mean percent reduction (improvement) from baseline in the UPDRS Motor Score. In this study 241 patients were enrolled. At the end of the 6-month study, patients treated with ropinirole hydrochloride had 22% improvement in motor score, compared with a 4% worsening in the placebo group (p<0.001).
Statistically significant differences in UPDRS motor score improvement between ropinirole hydrochloride and placebo were seen after 12 weeks of treatment.
Study in Patients With Advanced Parkinson’s Disease (With L-dopa):
This double-blind, randomized, placebo-controlled, 6-month trial evaluated 148 patients (Hoehn & Yahr II-IV) who were not adequately controlled on L-dopa. Patients in this study had a mean disease duration of approximately 9 years, had been exposed to L-dopa for approximately 7 years, and had experienced “on-off” periods with L-dopa therapy. Patients previously receiving stable doses of selegiline, amantadine, and/or anticholinergic agents could continue on these agents during the study. Patients were started at a dose of 0.25 mg 3 times daily of ropinirole hydrochloride and titrated upward by weekly intervals until an optimal therapeutic response was achieved. The maximum dose of study medication was 8 mg 3 times daily. All patients had to be titrated to at least a dose of 2.5 mg 3 times daily. Patients could then be maintained on this dose level or higher for the remainder of the study. Once a dose of 2.5 mg 3 times daily was achieved, patients underwent a mandatory reduction in their L-dopa dose, to be followed by additional mandatory reductions with continued escalation of the dose of ropinirole hydrochloride. Reductions in the dosage of L-dopa were also allowed if patients experienced adverse events that the investigator considered related to dopaminergic therapy. The mean dose attained at study endpoint was 16.3 mg/day. The primary outcome was the proportion of responders, defined as patients who were able both to achieve a decrease (compared to baseline) of at least 20% in their L-dopa dose and a decrease of at least 20% in the proportion of the time awake in the “off” condition (a period of time during the day when patients are particularly immobile), as determined by patient diary. In addition, the mean percent change from baseline in daily L-dopa dose was examined.
At the end of 6 months, 28% of patients treated with ropinirole hydrochloride were classified as responders (based on combined endpoint) while 11% of patients treated with placebo were responders (p = 0.02). Based on the protocol-mandated reductions in L-dopa dosage with escalating doses of ropinirole hydrochloride, patients treated with ropinirole hydrochloride had a 19.4% mean reduction in L-dopa dose while patients treated with placebo had a 3% reduction (p<0.001). L-dopa dosage reduction was also allowed during the study if dyskinesias or other dopaminergic effects occurred. Overall, reduction of L-dopa dose was sustained in 87% of patients treated with ropinirole hydrochloride and in 57% of patients on placebo. On average, the L-dopa dose was reduced by 31% in patients treated with ropinirole hydrochloride.
The mean number of “off” hours per day during baseline was 6.4 hours for patients treated with ropinirole hydrochloride and 7.3 hours for patients treated with placebo. At the end of the 6-month study, patients treated with ropinirole hydrochloride had a mean of 4.9 hours per day of “off” time, while placebo-treated patients had a mean of 6.4 hours per day of “off” time.
Restless Legs Syndrome (RLS):
The effectiveness of ropinirole hydrochloride in the treatment of RLS was demonstrated in randomized, double-blind, placebo-controlled studies in adults diagnosed with RLS using the International Restless Legs Syndrome Study Group diagnostic criteria (see INDICATIONS AND USAGE). Patients were required to have a history of a minimum of 15 RLS episodes/month during the previous month and a total score of ≥15 on the International RLS Rating Scale (IRLS scale) at baseline. Patients with RLS secondary to other conditions (e.g., pregnancy, renal failure, and anemia) were excluded. All studies employed flexible dosing, with patients initiating therapy at 0.25 mg ropinirole hydrochloride once daily. Patients were titrated based on clinical response and tolerability over 7 weeks to a maximum of 4 mg once daily. All doses were taken between 1 and 3 hours before bedtime.
A variety of measures were used to assess the effects of treatment, including the IRLS Scale and Clinical Global Impression-Global Improvement (CGI-I) scores. The IRLS Scale contains 10 items designed to assess the severity of sensory and motor symptoms, sleep disturbance, daytime somnolence, and impact on activities of daily living and mood associated with RLS. The range of scores is 0 to 40, with 0 being absence of RLS symptoms and 40 the most severe symptoms. Three of the controlled studies utilized the change from baseline in the IRLS Scale at the week 12 endpoint as the primary efficacy outcome.
Three hundred eighty patients were randomized to receive ropinirole hydrochloride (n = 187) or placebo (n = 193) in a US study; 284 were randomized to receive either ropinirole hydrochloride (n = 146) or placebo (n = 138) in a multinational study (excluding US); and 267 patients were randomized to ropinirole hydrochloride (n = 131) or placebo (n = 136) in a multinational study (including US). Across the 3 studies, the mean duration of RLS was 16 to 22 years (range of 0 to 65 years), mean age was approximately 54 years (range of 18 to 79 years), and approximately 61% were women. The mean dose at week 12 was approximately 2 mg/day for the 3 studies.
In all 3 studies, a statistically significant difference between the treatment group receiving ropinirole hydrochloride and the treatment group receiving placebo was observed at week 12 for both the mean change from baseline in the IRLS Scale total score and the percentage of patients rated as responders (much improved or very much improved) on the CGI-I (see Table 1).
| Ropinirole Hydrochloride | Placebo | p-value | |
| Mean Change in IRLS score at Week 12 | |||
| US study | -13.5 | -9.8 | p<0.0001 |
| Multinational study (excluding US) | -11.0 | -8.0 | p=0.0036 |
| Multinational study (including US) | -11.2 | -8.7 | p=0.0197 |
| Percent responders on CGI-I at Week 12 | |||
| US study | 73.3% | 56.5% | p=0.0006 |
| Multinational study (excluding US) | 53.4% | 40.9% | p=0.0416 |
| Multinational study (including US) | 59.5% | 39.6% | p=0.0010 |
Long-term maintenance of efficacy in the treatment of RLS was demonstrated in a 36-week study. Following a 24-week single-blind treatment phase (flexible doses of ropinirole hydrochloride of 0.25 to 4 mg once daily), patients who were responders (defined as a decrease of >6 points on the IRLS Scale total score relative to baseline) were randomized in double-blind fashion to placebo or continuation of ropinirole hydrochloride for an additional 12 weeks. Relapse was defined as an increase of at least 6 points on the IRLS Scale total score to a total score of at least 15, or withdrawal due to lack of efficacy. For patients who were responders at week 24, the mean dose of ropinirole was 2 mg (range 0.25 to 4 mg). Patients continued on ropinirole hydrochloride demonstrated a significantly lower relapse rate compared with patients randomized to placebo (32.6% vs 57.8%, p = 0.0156).
ROPINIROLE INDICATIONS AND USAGE
Parkinson’s Disease:
Ropinirole tablets USP are indicated for the treatment of the signs and symptoms of idiopathic Parkinson’s disease.
The effectiveness of ropinirole hydrochloride was demonstrated in randomized, controlled trials in patients with early Parkinson’s disease who were not receiving concomitant L-dopa therapy as well as in patients with advanced disease on concomitant L-dopa (see CLINICAL PHARMACOLOGY: Clinical Trials).
Restless Legs Syndrome:
Ropinirole tablets USP are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS).
Key diagnostic criteria for RLS are: an urge to move the legs usually accompanied or caused by uncomfortable and unpleasant leg sensations; symptoms begin or worsen during periods of rest or inactivity such as lying or sitting; symptoms are partially or totally relieved by movement such as walking or stretching at least as long as the activity continues; and symptoms are worse or occur only in the evening or night. Difficulty falling asleep may frequently be associated with moderate-to-severe RLS.
ROPINIROLE CONTRAINDICATIONS
Ropinirole hydrochloride is contraindicated for patients known to have hypersensitivity to the product.
WARNINGS
Falling Asleep During Activities of Daily Living: Patients treated with ropinirole hydrochloride have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence while on ropinirole hydrochloride, some perceived that they had no warning signs such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events have been reported as late as 1 year after initiation of treatment.
In controlled clinical trials, somnolence was a common occurrence in patients receiving ropinirole hydrochloride and is more frequent in Parkinson's disease (up to 40% ropinirole hydrochloride, 6% placebo) than in Restless Legs Syndrome (12% ropinirole hydrochloride, 6% placebo). Many clinical experts believe that falling asleep while engaged in activities of daily living always occurs in a setting of preexisting somnolence, although patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with ropinirole hydrochloride, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with ropinirole hydrochloride such as concomitant sedating medications, the presence of sleep disorders (other than Restless Legs Syndrome), and concomitant medications that increase ropinirole plasma levels (e.g., ciprofloxacin—see PRECAUTIONS: Drug Interactions). If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.), ropinirole hydrochloride should ordinarily be discontinued. (See DOSAGE AND ADMINISTRATION for guidance in discontinuing ropinirole hydrochloride.) If a decision is made to continue ropinirole hydrochloride, patients should be advised to not drive and to avoid other potentially dangerous activities. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Syncope:
Syncope, sometimes associated with bradycardia, was observed in association with ropinirole in both Parkinson’s disease patients and RLS patients. In the 2 double-blind, placebo-controlled studies of ropinirole hydrochloride in patients with Parkinson’s disease who were not being treated with L-dopa, 11.5% (18 of 157) of patients on ropinirole hydrochloride had syncope compared to 1.4% (2 of 147) of patients on placebo. Most of these cases occurred more than 4 weeks after initiation of therapy with ropinirole hydrochloride, and were usually associated with a recent increase in dose.
Of 208 patients being treated with both L-dopa and ropinirole hydrochloride in placebo-controlled advanced Parkinson’s disease trials, there were reports of syncope in 6 (2.9%) compared to 2 of 120 (1.7%) of placebo/L-dopa patients.
In patients with RLS, of 496 patients treated with ropinirole hydrochloride in 12-week placebo-controlled trials, there were reports of syncope in 5 (1.0%) compared with 1 of 500 (0.2%) patients treated with placebo.
Because the studies of ropinirole hydrochloride excluded patients with significant cardiovascular disease, it is not known to what extent the estimated incidence figures apply to either Parkinson’s disease or RLS patients in clinical practice. Therefore, patients with severe cardiovascular disease should be treated with caution.
Two of 47 Parkinson’s disease patient volunteers enrolled in phase 1 studies had syncope following a 1-mg dose. In 2 studies in RLS patients that used a forced titration regimen and orthostatic challenge with intensive blood pressure monitoring, 1 of 55 RLS patients treated with ropinirole hydrochloride compared with 0 of 27 patients receiving placebo reported syncope. In phase 1 studies including 110 healthy volunteers, 1 patient developed hypotension, bradycardia, and sinus arrest of 26 seconds accompanied by syncope; the patient recovered spontaneously without intervention. One other healthy volunteer reported syncope.
Symptomatic Hypotension:
Dopamine agonists, in clinical studies and clinical experience, appear to impair the systemic regulation of blood pressure, with resulting postural hypotension, especially during dose escalation. Parkinson’s disease patients, in addition, appear to have an impaired capacity to respond to a postural challenge. For these reasons, Parkinson’s patients being treated with dopaminergic agonists ordinarily (1) require careful monitoring for signs and symptoms of postural hypotension, especially during dose escalation, and (2) should be informed of this risk (see PRECAUTIONS: Information for Patients).
Although the clinical trials were not designed to systematically monitor blood pressure, there were individual reported cases of postural hypotension in early Parkinson’s disease (without L-dopa) in patients treated with ropinirole hydrochloride. Most of these cases occurred more than 4 weeks after initiation of therapy with ropinirole hydrochloride and were usually associated with a recent increase in dose.
In 12-week placebo-controlled trials of patients with RLS, the adverse event orthostatic hypotension was reported by 4 of 496 patients (0.8%) treated with ropinirole hydrochloride compared with 2 of 500 patients (0.4%) receiving placebo.
In two phase 2 studies in patients with RLS that used a forced-titration regimen and orthostatic challenges with intensive blood pressure monitoring, 14 of 55 patients (25%) receiving ropinirole hydrochloride experienced an adverse event of hypotension or postural hypotension. As described above, one additional patient was noted to have an episode of vasovagal syncope (although no blood pressure recording was documented). None of the 27 patients receiving placebo had a similar adverse event. In these studies, 11 of the 55 patients (20%) receiving ropinirole hydrochloride and 3 of the 26 patients (12%) who had post-dose blood pressure assessments following placebo, experienced an orthostatic blood pressure decrease of at least 40 mm Hg systolic and/or at least 20 mm Hg diastolic; not all of these changes were associated with clinical symptoms. Except for its forced nature these studies used a similar titration schedule as those in the phase 3 efficacy trials.
In phase 1 studies of ropinirole hydrochloride that included 110 healthy volunteers, 9 subjects had documented symptomatic postural hypotension. These episodes appeared mainly at doses above 0.8 mg and these doses are higher than the starting doses recommended for either Parkinson’s disease patients or RLS patients. In 8 of these 9 individuals, the hypotension was accompanied by bradycardia, but did not develop into syncope (see Syncope subsection). None of these events resulted in death or hospitalization.
One of 47 Parkinson’s disease patient volunteers enrolled in phase 1 studies had documented hypotension following a 2-mg dose on 2 occasions.
Hallucinations:
In double-blind, placebo-controlled, early-therapy studies in patients with Parkinson’s disease who were not treated with L-dopa, 5.2% (8 of 157) of patients treated with ropinirole hydrochloride reported hallucinations, compared to 1.4% of patients on placebo (2 of 147). Among those patients receiving both ropinirole hydrochloride and L-dopa in advanced Parkinson’s disease (with L-dopa) studies, 10.1% (21 of 208) were reported to experience hallucinations, compared to 4.2% (5 of 120) of patients treated with placebo and L-dopa.
Hallucinations were of sufficient severity to cause discontinuation of treatment in 1.3% of the early Parkinson’s disease (without L-dopa) patients and 1.9% of the advanced Parkinson’s disease (with L-dopa) patients, compared to 0% and 1.7% of placebo patients, respectively.
In patients with RLS, hallucinations were reported by 0% of patients treated with ropinirole hydrochloride (0 of 496) compared with 0.2% of patients who received placebo (1 of 500) in the 12-week placebo-controlled trials; in premarketing long-term open-label studies, 0.5% of patients reported hallucinations during therapy with ropinirole hydrochloride (2 of 390) but did not discontinue treatment and symptoms resolved.
PRECAUTIONS
General
Dyskinesia:
Ropinirole hydrochloride may potentiate the dopaminergic side effects of L-dopa and may cause and/or exacerbate preexisting dyskinesia in patients treated with L-dopa for Parkinson's disease. Decreasing the dose of L-dopa may ameliorate this side effect.
Renal Impairment:
No dosage adjustment is needed in patients with mild to moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The use of ropinirole hydrochloride in patients with severe renal impairment has not been studied.
Hepatic Impairment:
The pharmacokinetics of ropinirole have not been studied in patients with hepatic impairment. Since patients with hepatic impairment may have higher plasma levels and lower clearance, ropinirole hydrochloride should be titrated with caution in these patients.
Events Reported With Dopaminergic Therapy: Withdrawal-Emergent Hyperpyrexia and Confusion:
Although not reported with ropinirole hydrochloride, a symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in anti-Parkinsonian therapy.
Fibrotic Complications
Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, pericarditis, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur.
Although these adverse events are believed to be related to the ergoline structure of these compounds, whether other, nonergot-derived dopamine agonists can cause them is unknown.
A small number of reports have been received of possible fibrotic complications, including pleural effusion, pleural fibrosis, interstitial lung disease, and cardiac valvulopathy, in the development program and postmarketing experience for ropinirole hydrochloride. While the evidence is not sufficient to establish a causal relationship between ropinirole hydrochloride and these fibrotic complications, a contribution of ropinirole hydrochloride cannot be completely ruled out in rare cases.
Melanoma:
Epidemiologic studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using ropinirole hydrochloride for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Augmentation and Rebound in RLS:
Reports in the literature indicate treatment of RLS with dopaminergic medications can result in a worsening of symptoms in the early morning hours, referred to as rebound. Augmentation has also been described during therapy for RLS. Augmentation refers to the earlier onset of symptoms in the evening (or even the afternoon), increase in symptoms, and spread of symptoms to involve other extremities. The controlled trials of ropinirole hydrochloride in patients with RLS excluded patients with augmentation and rebound and were generally not of sufficient duration to capture these phenomena. The frequency of augmentation and/or rebound after longer use of ropinirole hydrochloride and the appropriate management of these events, have not been evaluated in controlled clinical trials.
Retinal Pathology:
Albino Rats:
Retinal degeneration was observed in albino rats in the 2-year carcinogenicity study at all doses tested (equivalent to 0.6 to 20 times the maximum recommended human dose on a mg/m2 basis), but was statistically significant at the highest dose (50 mg/kg/day). Additional studies to further evaluate the specific pathology (e.g., loss of photoreceptor cells) have not been performed. Similar changes were not observed in a 2-year carcinogenicity study in albino mice or in rats or monkeys treated for 1 year. The potential significance of this effect in humans has not been established, but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding) may be involved.
Human
In order to evaluate the effect of ropinirole hydrochloride in humans, ocular electroretinogram (ERG) assessments were conducted during a 2-year, double-blind, multicenter, flexible dose, L-dopa controlled clinical study of ropinirole hydrochloride in patients with Parkinson's disease. A total of 156 patients (78 on ropinirole, mean dose 11.9 mg/day and 78 on L-dopa, mean dose 555.2 mg/day) were evaluated for evidence of retinal dysfunction through electroretinograms. There was no clinically meaningful difference between the treatment groups in retinal function over the duration of the study.
Binding to Melanin:
Ropinirole hydrochloride binds to melanin-containing tissues (i.e., eyes, skin) in pigmented rats. After a single dose, long-term retention of drug was demonstrated, with a half-life in the eye of 20 days. It is not known if ropinirole hydrochloride accumulates in these tissues over time.
Information for Patients
Physicians should instruct their patients to read the Patient Information leaflet before starting therapy with ropinirole hydrochloride and to reread it upon prescription renewal for new information regarding the use of ropinirole hydrochloride.
Patients should be instructed to take ropinirole hydrochloride only as prescribed. If a dose is missed, patients should be advised not to double their next dose.
Ropinirole hydrochloride can be taken with or without food. Patients may be advised that taking ropinirole hydrochloride with food may reduce the occurrence of nausea. However, this has not been established in controlled clinical trials.
Patients should be advised that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Hypotension and/or orthostatic symptoms may occur more frequently during initial therapy or with an increase in dose at any time (cases have been seen after weeks of treatment). Accordingly, patients should be cautioned against rising rapidly after sitting or lying down, especially if they have been doing so for prolonged periods, and especially at the initiation of treatment with ropinirole hydrochloride.
Patients should be alerted to the potential sedating effects associated with ropinirole hydrochloride, including somnolence and the possibility of falling asleep while engaged in activities of daily living. Since somnolence is a frequent adverse event with potentially serious consequences, patients should neither drive a car nor engage in other potentially dangerous activities until they have gained sufficient experience with ropinirole hydrochloride to gauge whether or not it affects their mental and/or motor performance adversely. Patients should be advised that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician.
Because of possible additive effects, caution should be advised when patients are taking other sedating medications or alcohol in combination with ropinirole hydrochloride and when taking concomitant medications that increase plasma levels of ropinirole (e.g., ciprofloxacin).
Because of the possible additive sedative effects, caution should also be used when patients are taking alcohol or other CNS depressants (e.g., benzodiazepines, antipsychotics, antidepressants, etc.) in combination with ropinirole hydrochloride.
Patients should be informed they may experience hallucinations (unreal visions, sounds, or sensations) while taking ropinirole hydrochloride. These were uncommon in patients taking ropinirole hydrochloride for Restless Legs Syndrome. The risk is greater in patients with Parkinson's disease; the elderly are at greater risk than younger patients with Parkinson's disease; and the risk is greater in patients who are taking ropinirole hydrochloride with L-dopa, or taking higher doses of ropinirole hydrochloride.
Impulse Control Symptoms Including Compulsive Behaviors:
There have been reports of patients experiencing intense urges to gamble, increased sexual urges, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson’s disease, including ropinirole hydrochloride. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges or other urges while being treated with ropinirole hydrochloride. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges or other intense urges while taking ropinirole hydrochloride. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking ropinirole hydrochloride.
Because of the possibility that ropinirole may be excreted in breast milk, patients should be advised to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
Because ropinirole has been shown to have adverse effects on embryo-fetal development, including teratogenic effects, in animals, and because experience in humans is limited, patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy (see PRECAUTIONS: Pregnancy).
Drug Interactions:
P Interaction:
In vitro metabolism studies showed that CYP1A2 was the major enzyme responsible for the metabolism of ropinirole. There is thus the potential for substrates or inhibitors of this enzyme when coadministered with ropinirole to alter its clearance. Therefore, if therapy with a drug known to be a potent inhibitor of CYP1A2 is stopped or started during treatment with ropinirole hydrochloride, adjustment of the dose of ropinirole hydrochloride may be required.
L-dopa:
Co-administration of carbidopa + L-dopa (SINEMET® 10/100 mg twice daily) with ropinirole (2 mg 3 times daily) had no effect on the steady-state pharmacokinetics of ropinirole (n = 28 patients). Oral administration of ropinirole hydrochloride 2 mg 3 times daily increased mean steady state Cmax of L-dopa by 20%, but its AUC was unaffected (n = 23 patients).
Digoxin
Co-administration of ropinirole hydrochloride (2 mg 3 times daily) with digoxin (0.125 to 0.25 mg once daily) did not alter the steady-state pharmacokinetics of digoxin in 10 patients.
Theophylline:
Administration of theophylline (300 mg twice daily, a substrate of CYP1A2) did not alter the steady-state pharmacokinetics of ropinirole (2 mg 3 times daily) in 12 patients with Parkinson’s disease. Ropinirole (2 mg 3 times daily) did not alter the pharmacokinetics of theophylline (5 mg/kg IV) in 12 patients with Parkinson’s disease.
Ciprofloxacin:
Co-administration of ciprofloxacin (500 mg twice daily), an inhibitor of CYP1A2, with ropinirole (2 mg 3 times daily) increased ropinirole AUC by 84% on average and Cmax by 60% (n = 12 patients).
Estrogens:
Population pharmacokinetic analysis revealed that estrogens (mainly ethinylestradiol: intake 0.6 to 3 mg over 4-month to 23-year period) reduced the oral clearance of ropinirole by 36% in 16 patients. Dosage adjustment may not be needed for ropinirole hydrochloride in patients on estrogen therapy because patients must be carefully titrated with ropinirole to tolerance or adequate effect. However, if estrogen therapy is stopped or started during treatment with ropinirole hydrochloride, then adjustment of the dose of ropinirole hydrochloride may be required.
Dopamine Antagonists:
Since ropinirole is a dopamine agonist, it is possible that dopamine antagonists such as neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide may diminish the effectiveness of ropinirole hydrochloride. Patients with major psychotic disorders treated with neuroleptics should only be treated with dopamine agonists if the potential benefits outweigh the risks.
Population analysis showed that commonly administered drugs, e.g., selegiline, amantadine, tricyclic antidepressants, benzodiazepines, ibuprofen, thiazides, antihistamines, and anticholinergics, did not affect the oral clearance of ropinirole.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in Charles River CD-1 mice at doses of 5, 15, and 50 mg/kg/day and in Sprague-Dawley rats at doses of 1.5, 15, and 50 mg/kg/day (top doses equivalent to 10 and 20 times, respectively, the maximum recommended human dose of 24 mg/day on a mg/m2basis). In the male rat, there was a significant increase in testicular Leydig cell adenomas at all doses tested, i.e., ≥1.5 mg/kg (0.6 times the maximum recommended human dose on a mg/m2 basis). This finding is of questionable significance because the endocrine mechanisms believed to be involved in the production of Leydig cell hyperplasia and adenomas in rats are not relevant to humans. In the female mouse, there was an increase in benign uterine endometrial polyps at a dose of 50 mg/kg/day (10 times the maximum recommended human dose on a mg/m2 basis).
Ropinirole was not mutagenic or clastogenic in the in vitro Ames test, the in vitro chromosome aberration test in human lymphocytes, the in vitro mouse lymphoma (L1578Y cells) assay, and the in vivo mouse micronucleus test.
When administered to female rats prior to and during mating and throughout pregnancy, ropinirole caused disruption of implantation at doses of 20 mg/kg/day (8 times the maximum recommended human dose on a mg/m2 basis) or greater. This effect is thought to be due to the prolactin-lowering effect of ropinirole. In humans, chorionic gonadotropin, not prolactin, is essential for implantation. In rat studies using low doses (5 mg/kg) during the prolactin-dependent phase of early pregnancy (gestation days 0 to 8), ropinirole did not affect female fertility at dosages up to 100 mg/kg/day (40 times the maximum recommended human dose on a mg/m2 basis). No effect on male fertility was observed in rats at dosages up to 125 mg/kg/day (50 times the maximum recommended human dose on a mg/m2 basis).
Pregnancy
Pregnancy Category C. In animal reproduction studies, ropinirole has been shown to have adverse effects on embryo-fetal development, including teratogenic effects. Ropinirole given to pregnant rats during organogenesis (20 mg/kg on gestation days 6 and 7 followed by 20, 60, 90, 120, or 150 mg/kg on gestation days 8 through 15) resulted in decreased fetal body weight at 60 mg/kg/day, increased fetal death at 90 mg/kg/day, and digital malformations at 150 mg/kg/day (24, 36, and 60 times the maximum recommended clinical dose on a mg/m2 basis, respectively). The combined administration of ropinirole (10 mg/kg/day, 8 times the maximum recommended human dose on a mg/m2 basis) and L-dopa (250 mg/kg/day) to pregnant rabbits during organogenesis produced a greater incidence and severity of fetal malformations (primarily digit defects) than were seen in the offspring of rabbits treated with L-dopa alone. No indication of an effect on development of the conceptus was observed in rabbits when a maternally toxic dose of ropinirole was administered alone (20 mg/kg/day, 16 times the maximum recommended human dose on a mg/m2 basis). In a perinatal-postnatal study in rats, 10 mg/kg/day (4 times the maximum recommended human dose on a mg/m2 basis) of ropinirole impaired growth and development of nursing offspring and altered neurological development of female offspring.
There are no adequate and well-controlled studies using ropinirole hydrochloride in pregnant women. Ropinirole hydrochloride should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus.
Nursing Mothers
Ropinirole hydrochloride inhibits prolactin secretion in humans and could potentially inhibit lactation.
Studies in rats have shown that ropinirole hydrochloride and/or its metabolite(s) is excreted in breast milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ropinirole hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established.
ROPINIROLE ADVERSE REACTIONS
Parkinson’s Disease:
During the premarketing development of ropinirole hydrochloride, patients received ropinirole hydrochloride either without L-dopa (early Parkinson’s disease studies) or as concomitant therapy with L-dopa (advanced Parkinson’s disease studies). Because these 2 populations may have differential risks for various adverse events, this section will, in general, present adverse event data for these 2 populations separately.
Early Parkinson’s Disease (Without L-dopa):
The most commonly observed adverse events (>5%) in the double-blind, placebo-controlled early Parkinson’s disease trials associated with the use of ropinirole hydrochloride (n = 157) not seen at an equivalent frequency among the placebo-treated patients (n = 147) were, in order of decreasing incidence: nausea, dizziness, somnolence, headache, vomiting, syncope, fatigue, dyspepsia, viral infection, constipation, pain, increased sweating, asthenia, dependent/leg edema, orthostatic symptoms, abdominal pain, pharyngitis, confusion, hallucinations, urinary tract infections, and abnormal vision.
Approximately 24% of 157 patients treated with ropinirole hydrochloride who participated in the double-blind, placebo-controlled early Parkinson’s disease (without L-dopa) trials discontinued treatment due to adverse events compared to 13% of 147 patients who received placebo. The adverse events most commonly causing discontinuation of treatment by patients treated with ropinirole hydrochloride were: nausea (6.4%), dizziness (3.8%), aggravated Parkinson’s disease (1.3%), hallucinations (1.3%), somnolence (1.3%), vomiting (1.3%), and headache (1.3%). Of these, hallucinations appear to be dose-related. While other adverse events leading to discontinuation may be dose-related, the titration design utilized in these trials precluded an adequate assessment of the dose response. For example, in the larger of the 2 trials described in CLINICAL PHARMACOLOGY: Clinical Trials, the difference in the rate of discontinuations emerged only after 10 weeks of treatment, suggesting, although not proving, that the effect could be related to dose.
Adverse Event Incidence in Controlled Clinical Studies:
Table 2 lists treatment-emergent adverse events that occurred in ≥2% of patients with early Parkinson’s disease (without L-dopa) treated with ropinirole hydrochloride participating in the double-blind, placebo-controlled studies and were numerically more common in the group treated with ropinirole hydrochloride. In these studies, either ropinirole hydrochloride or placebo was used as early therapy (i.e., without L-dopa).
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse-events incidence rate in the population studied.
| * Patients may have reported multiple adverse experiences during the study or at discontinuation; thus, patients may be included in more than one category. | ||
| Adverse Experience |
Ropinirole Hydrochloride (n = 157) (%) |
Placebo (n = 147) (%) |
|
Autonomic nervous system Flushing Dry mouth Increased sweating |
3 5 6 |
1 3 4 |
|
Body as a whole Asthenia Chest pain Dependent edema Leg edema Fatigue Malaise Pain |
6 4 6 7 11 3 8 |
1 2 3 1 4 1 4 |
|
Cardiovascular general Hypertension Hypotension Orthostatic symptoms Syncope |
5 2 6 12 |
3 0 5 1 |
|
Central/peripheral nervous system Dizziness Hyperkinesia Hypesthesia Vertigo |
40 2 4 2 |
22 1 2 0 |
|
Gastrointestinal system Abdominal pain Anorexia Dyspepsia Flatulence Nausea Vomiting |
6 4 10 3 60 12 |
3 1 5 1 22 7 |
|
Heart rate/rhythm Extrasystoles Atrial fibrillation Palpitation Tachycardia |
2 2 3 2 |
1 0 2 0 |
|
Metabolic/nutritional Increased alkaline phosphatase |
3 | 1 |
|
Psychiatric Amnesia Impaired concentration Confusion Hallucination Somnolence Yawning |
3 2 5 5 40 3 |
1 0 1 1 6 0 |
|
Reproductive male Impotence |
3 | 1 |
|
Resistance mechanism Viral infection |
11 | 3 |
|
Respiratory system Bronchitis Dyspnea Pharyngitis Rhinitis Sinusitis |
3 3 6 4 4 |
1 0 4 3 3 |
|
Urinary system Urinary tract infection |
5 | 4 |
|
Vascular extracardiac Peripheral ischemia |
3 | 0 |
|
Vision Eye abnormality Abnormal vision Xerophthalmia |
3 6 2 |
1 3 0 |
Other events reported by 1% or more of early Parkinson’s disease (without L-dopa) patients treated with ropinirole hydrochloride, but that were equally or more frequent in the placebo group, were: headache, upper respiratory infection, insomnia, arthralgia, tremor, back pain, anxiety, dyskinesias, aggravated Parkinsonism, depression, falls, myalgia, leg cramps, paresthesias, nervousness, diarrhea, arthritis, hot flushes, weight loss, rash, cough, hyperglycemia, muscle spasm, arthrosis, abnormal dreams, dystonia, increased salivation, bradycardia, gout, basal cell carcinoma, gingivitis, hematuria, and rigors.
Among the treatment-emergent adverse events in patients treated with ropinirole hydrochloride, hallucinations appear to be dose-related.
The incidence of adverse events was not materially different between women and men.
Advanced Parkinson’s Disease (With L-dopa):
The most commonly observed adverse events (>5%), in the double-blind, placebo-controlled advanced Parkinson’s disease (with L-dopa) trials associated with the use of ropinirole hydrochloride (n = 208) as an adjunct to L-dopa not seen at an equivalent frequency among the placebo-treated patients (n = 120) were, in order of decreasing incidence: dyskinesias, nausea, dizziness, aggravated Parkinsonism, somnolence, headache, insomnia, injury, hallucinations, falls, abdominal pain, upper respiratory infection, confusion, increased sweating, vomiting, viral infection, increased drug level, arthralgia, tremor, anxiety, urinary tract infection, constipation, dry mouth, pain, hypokinesia, and paresthesia.
Approximately 24% of 208 patients who received ropinirole hydrochloride in the double-blind, placebo-controlled advanced Parkinson’s disease (with L-dopa) trials discontinued treatment due to adverse events compared to 18% of 120 patients who received placebo. The events most commonly (≥1%) causing discontinuation of treatment by patients treated with ropinirole hydrochloride were: dizziness (2.9%), dyskinesias (2.4%), vomiting (2.4%), confusion (2.4%), nausea (1.9%), hallucinations (1.9%), anxiety (1.9%), and increased sweating (1.4%). Of these, hallucinations and dyskinesias appear to be dose-related.
Adverse Event Incidence in Controlled Clinical Studies:
Table 3 lists treatment-emergent adverse events that occurred in ≥2% of patients with advanced Parkinson’s disease (with L-dopa) treated with ropinirole hydrochloride who participated in the double-blind, placebo-controlled studies and were numerically more common in the group treated with ropinirole hydrochloride. In these studies, either ropinirole hydrochloride or placebo was used as an adjunct to L-dopa. Adverse events were usually mild or moderate in intensity.
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse events incidence rate in the population studied.
| * Patients may have reported multiple adverse experiences during the study or at discontinuation; thus, patients may be included in more than one category. | ||
| Adverse Experience |
Ropinirole Hydrochloride (n = 208) (%) |
Placebo (n = 120) (%) |
|
Autonomic nervous system Dry mouth Increased sweating |
5 7 |
1 2 |
|
Body as a whole Increased drug level Pain |
7 5 |
3 3 |
|
Cardiovascular general Hypotension Syncope |
2 3 |
1 2 |
|
Central/peripheral nervous system Dizziness Dyskinesia Falls Headache Hypokinesia Paresis Paresthesia Tremor |
26 34 10 17 5 3 5 6 |
16 13 7 12 4 0 3 3 |
|
Gastrointestinal system Abdominal pain Constipation Diarrhea Dysphagia Flatulence Nausea Increased saliva Vomiting |
9 6 5 2 2 30 2 7 |
8 3 3 1 1 18 1 4 |
|
Metabolic/nutritional Weight decrease |
2 | 1 |
|
Musculoskeletal system Arthralgia Arthritis |
7 3 |
5 1 |
|
Psychiatric Amnesia Anxiety Confusion Abnormal dreaming Hallucinations Nervousness Somnolence |
5 6 9 3 10 5 20 |
1 3 2 2 4 3 8 |
|
Red blood cell Anemia |
2 | 0 |
|
Resistance mechanism Upper respiratory tract infection |
9 | 8 |
|
Respiratory system Dyspnea |
3 | 2 |
|
Urinary system Pyuria Urinary incontinence Urinary tract infection |
2 2 6 |
1 1 3 |
|
Vision Diplopia |
2 | 1 |
Other events reported by 1% or more of patients treated with both ropinirole hydrochloride and L-dopa, but equally or more frequent in the placebo/L-dopa group, were: myocardial infarction, orthostatic symptoms, virus infections, asthenia, dyspepsia, myalgia, back pain, depression, leg cramps, fatigue, rhinitis, chest pain, hematuria, vertigo, tinnitus, leg edema, hot flushes, abnormal gait, hyperkinesia, and pharyngitis.
Among the treatment-emergent adverse events in patients treated with ropinirole hydrochloride, hallucinations and dyskinesias appear to be dose-related.
Restless Legs Syndrome:
The most commonly observed adverse events (>5%) in the 12-week double-blind, placebo-controlled trials in the treatment of Restless Legs Syndrome with ropinirole hydrochloride (n = 496) and at least twice the rate for placebo-treated patients (n = 500) were, in order of decreasing incidence: nausea, somnolence, vomiting, dizziness, and fatigue (see Table 4). Occurrences of nausea in clinical trials were generally mild to moderate in intensity (see also DOSAGE AND ADMINISTRATION: General Dosing Considerations).
Approximately 5% of 496 patients treated with ropinirole hydrochloride who participated in the double-blind, placebo-controlled trials in the treatment of RLS discontinued treatment due to adverse events compared to 4% of 500 patients who received placebo. The adverse events most commonly causing discontinuation of treatment by patients treated with ropinirole hydrochloride were: nausea (1.6%), dizziness (0.8%), and headache (0.8%).
Adverse Event Incidence in Controlled Clinical Studies:
Table 4 lists treatment-emergent adverse events that occurred in ≥2% of patients with RLS treated with ropinirole hydrochloride participating in the 12-week double-blind, placebo-controlled studies and were numerically more common in the group treated with ropinirole hydrochloride.
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse-events incidence rate in the population studied.
| Adverse Experience |
Ropinirole Hydrochloride n = 496 (%) |
Placebo n =500 (%) |
|
Ear and labyrinth disorders Vertigo |
2 | 1 |
|
Gastrointestinal disorders Nausea Vomiting Diarrhea Dyspepsia Dry mouth Abdominal pain upper |
40 11 5 4 3 3 |
8 2 3 3 2 1 |
|
General disorders and administration site conditions Fatigue Edema peripheral |
8 2 |
4 1 |
|
Infections and infestations Nasopharyngitis Influenza |
9 3 |
8 2 |
|
Musculoskeletal and connective tissue disorders Arthralgia Muscle cramps Pain in extremity |
4 3 3 |
3 2 2 |
|
Nervous system disorders Somnolence Dizziness Paresthesia |
12 11 3 |
6 5 1 |
|
Respiratory, thoracic, and mediastinal disorders Cough Nasal congestion |
3 2 |
2 1 |
|
Skin and subcutaneous tissue disorders Hyperhidrosis |
3 | 1 |
Other events reported by 2% or more of patients treated with ropinirole hydrochloride, but equally or more frequent in the placebo group, were headache, insomnia, restless legs syndrome, upper respiratory tract infection, back pain, and sinusitis.
Other Adverse Events Observed During All Phase 2/3 Clinical Trials for Parkinson’s Disease:
Ropinirole hydrochloride has been administered to 1,599 individuals in clinical trials. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using modified WHOART dictionary terminology. These categories are used in the listing below. The frequencies presented represent the proportion of the 1,599 individuals exposed to ropinirole hydrochloride who experienced events of the type cited on at least 1 occasion while receiving ropinirole hydrochloride. All reported events that occurred at least twice (or once for serious or potentially serious events), except those already listed above, trivial events, and terms too vague to be meaningful, are included without regard to determination of a causal relationship to ropinirole hydrochloride, except that events very unlikely to be drug-related have been deleted.
Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients and infrequent adverse events are those occurring in 1/100 to 1/1,000 patients and rare events are those occurring in fewer than 1/1,000 patients.
Body as a Whole: Infrequent: Cellulitis, peripheral edema, fever, influenza-like symptoms, enlarged abdomen, precordial chest pain, and generalized edema. Rare : Ascites.
Cardiovascular: Infrequent: Cardiac failure, bradycardia, tachycardia, supraventricular tachycardia, angina pectoris, bundle branch block, cardiac arrest, cardiomegaly, aneurysm, mitral insufficiency. Rare: Ventricular tachycardia.
Central/Peripheral Nervous System: Frequent: Neuralgia. Infrequent: Involuntary muscle contractions, hypertonia, dysphonia, abnormal coordination, extrapyramidal disorder, migraine, choreoathetosis, coma, stupor, aphasia, convulsions, hypotonia, peripheral neuropathy, paralysis. Rare: Grand mal convulsions, hemiparesis, hemiplegia.
Endocrine: Infrequent: Hypothyroidism, gynecomastia, hyperthyroidism. Rare: Goiter, SIADH.
Gastrointestinal: Infrequent: Increased hepatic enzymes, bilirubinemia, cholecystitis, cholelithiasis colitis, dysphagia, periodontitis, fecal incontinence, gastroesophageal reflux, hemorrhoids, toothache, eructation, gastritis, esophagitis, hiccups, diverticulitis, duodenal ulcer, gastric ulcer, melena, duodenitis, gastrointestinal hemorrhage, glossitis, rectal hemorrhage, pancreatitis, stomatitis and ulcerative stomatitis, tongue edema. Rare: Biliary pain, hemorrhagic gastritis, hematemesis, salivary duct obstruction.
Hematologic: Infrequent: Purpura, thrombocytopenia, hematoma, Vitamin B12 deficiency, hypochromic anemia, eosinophilia, leukocytosis, leukopenia, lymphocytosis, lymphopenia, lymphedema.
Metabolic/Nutritional: Frequent: Increased BUN. Infrequent: Hypoglycemia, increased alkaline phosphatase, increased LDH, weight increase, hyperphosphatemia, hyperuricemia, diabetes mellitus, glycosuria, hypokalemia, hypercholesterolemia, hyperkalemia, acidosis, hyponatremia, thirst, increased CPK, dehydration. Rare: Hypochloremia.
Musculoskeletal: Infrequent: Aggravated arthritis, tendonitis, osteoporosis, bursitis, polymyalgia rheumatica, muscle weakness, skeletal pain, torticollis. Rare: Dupuytren’s contracture requiring surgery.
Neoplasm: Infrequent: Malignant breast neoplasm. Rare: Bladder carcinoma, benign brain neoplasm, esophageal carcinoma, malignant laryngeal neoplasm, lipoma, rectal carcinoma, uterine neoplasm.
Psychiatric: Infrequent: Increased libido, agitation, apathy, impaired concentration, depersonalization, paranoid reaction, personality disorder, euphoria, delirium, dementia, delusion, emotional lability, decreased libido, manic reaction, somnambulism, aggressive reaction, neurosis. Rare: Suicide attempt.
Genitourinary: Infrequent: Amenorrhea, vaginal hemorrhage, penile disorder, prostatic disorder, balanoposthitis, epididymitis, perineal pain, dysuria, micturition frequency, albuminuria, nocturia, polyuria, renal calculus. Rare: Breast enlargement, mastitis, uterine hemorrhage, ejaculation disorder, Peyronie’s disease, pyelonephritis, acute renal failure, uremia.
Resistance Mechanism: Infrequent: Herpes zoster, otitis media, sepsis, abscess, herpes simplex, fungal infection, genital moniliasis.
Respiratory: Infrequent: Asthma, epistaxis, laryngitis, pleurisy, pulmonary edema.
Skin/Appendage: Infrequent: Pruritus, dermatitis, eczema, skin ulceration, alopecia, skin hypertrophy, skin discoloration, urticaria, fungal dermatitis, furunculosis, hyperkeratosis, photosensitivity reaction, psoriasis, maculopapular rash, psoriaform rash, seborrhea.
Special Senses: Infrequent: Tinnitus, earache, decreased hearing, abnormal lacrimation, conjunctivitis, blepharitis, glaucoma, abnormal accommodation, blepharospasm, eye pain, photophobia. Rare: Scotoma.
Vascular Extracardiac: Infrequent: Varicose veins, phlebitis, peripheral gangrene. Rare: Limb embolism, pulmonary embolism, gangrene, subarachnoid hemorrhage, deep thrombophlebitis, leg thrombophlebitis, thrombosis.
Falling Asleep During Activities of Daily Living: Patients treated with ropinirole hydrochloride have reported falling asleep while engaged in activities of daily living, including operation of a motor vehicle which sometimes resulted in accidents (see bolded WARNING).
Other Adverse Events Observed During Phase 2/3 Clinical Trials for RLS:
Ropinirole hydrochloride has been administered to 911 individuals in clinical trials. During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using MedDRA dictionary terminology. These categories are used in the listing below. The frequencies presented represent the proportion of the 911 individuals exposed to ropinirole hydrochloride who experienced events of the type cited on at least one occasion while receiving ropinirole hydrochloride. All reported events that occurred at least twice (or once for serious or potentially serious events), except those already listed, trivial events, and terms too vague to be meaningful, are included without regard to determination of a causal relationship to ropinirole hydrochloride, except that events very unlikely to be drug-related have been deleted.
Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients and infrequent adverse events are those occurring in 1/100 to 1/1,000 patients.
Blood and Lymphatic System Disorders: Infrequent: Anemia, lymphadenopathy.
Cardiac Disorders: Frequent: Palpitations. Infrequent: Acute coronary syndrome, angina pectoris, angina unstable, bradycardia, cardiac failure, cardiovascular disorder, coronary artery disease, myocardial infarction, sick sinus syndrome, tachycardia.
Congenital, Familial, and Genetic Disorders: Infrequent: Pigmented nevus.
Ear and Labyrinth Disorders: Infrequent: Ear pain, middle ear effusion, tinnitus.
Endocrine Disorders: Infrequent: Goiter, hypothyroidism.
Eye Disorders: Infrequent: Blepharitis, conjunctival hemorrhage, conjunctivitis, eye irritation, eye pain, keratoconjunctivitis sicca, vision blurred, visual acuity reduced, visual disturbance.
Gastrointestinal Disorders: Frequent: Abdominal pain, constipation, gastroesophageal reflux disease, stomach discomfort, toothache. Infrequent: Abdominal adhesions, abdominal discomfort, abdominal distension, abdominal pain lower, duodenal ulcer, dysphagia, eructation, flatulence, gastric disorder, gastric hemorrhage, gastric polyps, gastric ulcer, gastritis, gastrointestinal pain, hematemesis, hemorrhoids, hiatus hernia, intestinal obstruction, irritable bowel syndrome, loose stools, mouth ulceration, pancreatitis acute, peptic ulcer, rectal hemorrhage, reflux esophagitis.
General Disorders and Administration Site Conditions: Frequent: Asthenia, chest pain, influenza-like illness, rigors. Infrequent: Chest discomfort, feeling cold, feeling hot, hunger, lethargy, malaise, edema, pain, pyrexia.
Hepatobiliary Disorders: Infrequent: Cholecystitis, cholelithiasis, ischemic hepatitis.
Immune System Disorders: Infrequent: Hypersensitivity.
Infections and Infestations: Frequent: Bronchitis, gastroenteritis, gastroenteritis viral, lower respiratory tract infection, rhinitis, tooth abscess, urinary tract infection. Infrequent: Appendicitis, bacterial infection, bladder infection, bronchitis acute, candidiasis, cellulitis, cystitis, diarrhea infectious, diverticulitis, ear infection, folliculitis, fungal infection, gastrointestinal infection, herpes simplex, infected cyst, laryngitis, localized infection, mastitis, otitis externa, otitis media, pharyngitis, pneumonia, postoperative infection, respiratory tract infection, tonsillitis, tooth infection, vaginal candidiasis, vaginal infection, vaginal mycosis, viral infection, viral upper respiratory tract infection, wound infection.
Injury, Poisoning, and Procedural Complications: Infrequent: Concussion, lower limb fracture, post procedural hemorrhage, road traffic accident.
Investigations: Infrequent: Blood cholesterol increased, blood iron decreased, blood pressure increased, blood urine present, hemoglobin decreased, heart rate increased, protein urine present, weight decreased, weight increased.
Metabolism and Nutrition Disorders: Infrequent: Anorexia, decreased appetite, diabetes mellitus non-insulin-dependent, fluid retention, gout, hypercholesterolemia.
Musculoskeletal and Connective Tissue Disorders: Frequent: Muscle spasms, musculoskeletal stiffness, myalgia, neck pain, osteoarthritis, tendonitis. Infrequent: Arthritis, aseptic necrosis bone, bone pain, bone spur, bursitis, groin pain, intervertebral disc degeneration, intervertebral disc protrusion, joint stiffness, joint swelling, localized osteoarthritis, monoarthritis, muscle contracture, muscle tightness, muscle twitching, osteoporosis, rotator cuff syndrome, sacroiliitis, synovitis.
Neoplasms Benign, Malignant, and Unspecified: Infrequent: Anaplastic thyroid cancer, angiomyolipoma, basal cell carcinoma, breast cancer, gastric cancer, gastrointestinal stromal tumor, malignant melanoma, prostate cancer, skin papilloma, squamous cell carcinoma, uterine leiomyoma.
Nervous System Disorders: Frequent: Hypoesthesia, migraine. Infrequent: Amnesia, aphasia, ataxia, balance disorder, benign intracranial hypertension, burning sensation, carpal tunnel syndrome, disturbance in attention, dizziness postural, dysgeusia, dyskinesia, head discomfort, hyperesthesia, hypersomnia, lethargy, loss of consciousness, memory impairment, migraine with aura, migraine without aura, neuralgia, sciatica, sedation, sinus headache, sleep apnea syndrome, syncope vasovagal, tension headache, transient ischemic attack, tremor.
Psychiatric Disorders: Frequent: Anxiety, depression, irritability, sleep disorder. Infrequent: Abnormal dreams, agitation, bruxism, confusional state, depressed mood, disorientation, early morning awakening, libido decreased, loss of libido, mood swings, nervousness, nightmare, panic attack, stress symptoms, tension.
Renal and Urinary Disorders: Infrequent: Dysuria, hematuria, hypertonic bladder, micturition disorder, nephrolithiasis, nocturia, pollakiuria, proteinuria, urinary retention.
Reproductive System and Breast Disorders: Frequent: Erectile dysfunction. Infrequent: Breast cyst, dysmenorrhea, menorrhagia, pelvic peritoneal adhesions, postmenopausal hemorrhage, premenstrual syndrome, prostatitis.
Respiratory, Thoracic and Mediastinal Disorders: Frequent: Asthma, pharyngolaryngeal pain. Infrequent: Dry throat, dyspnea, epistaxis, hemoptysis, hoarseness, interstitial lung disease, nasal mucosal disorder, nasal polyps, respiratory tract congestion, rhinorrhea, sinus congestion, sneezing, wheezing, yawning.
Skin and Subcutaneous Tissue Disorders: Frequent: Night sweats, rash. Infrequent: Acne, actinic keratosis, alopecia, cold sweat, dermatitis, dermatitis allergic, dermatitis contact, eczema, exanthem, face edema, photosensitivity reaction, pruritus, psoriasis, rash pruritic, skin lesion, urticaria.
Vascular Disorders: Frequent: Hot flush, hypertension, hypotension. Infrequent: Atherosclerosis, circulatory collapse, flushing, hematoma, thrombosis, varicose vein.
Postmarketing Reports:
Psychiatric Disorders: Impulse control symptoms, pathological gambling, increased libido including hypersexuality.
DRUG ABUSE AND DEPENDENCE
Controlled Substance Class:
Ropinirole hydrochloride is not a controlled substance.
Physical and Psychological Dependence:
Animal studies and human clinical trials with ropinirole hydrochloride did not reveal any potential for drug-seeking behavior or physical dependence.
OVERDOSAGE
In the Parkinson's disease program, there have been patients who accidentally or intentionally took more than their prescribed dose of ropinirole. The largest overdose reported in the Parkinson's disease clinical trials was 435 mg taken over a 7-day period (62.1 mg/day). Of patients who received a dose greater than 24 mg/day, reported symptoms included adverse events commonly reported during dopaminergic therapy (nausea, dizziness), as well as visual hallucinations, hyperhidrosis, claustrophobia, chorea, palpitations, asthenia, and nightmares. Additional symptoms reported for doses of 24 mg or less or for overdoses of unknown amount included vomiting, increased coughing, fatigue, syncope, vasovagal syncope, dyskinesia, agitation, chest pain, orthostatic hypotension, somnolence, and confusional state.
Overdose Management:
It is anticipated that the symptoms of overdose with ropinirole hydrochloride will be related to its dopaminergic activity. General supportive measures are recommended. Vital signs should be maintained, if necessary. Removal of any unabsorbed material (e.g., by gastric lavage) should be considered.
ROPINIROLE DOSAGE AND ADMINISTRATION
General Dosing Considerations for Parkinson's Disease and RLS:
Ropinirole tablets USP can be taken with or without food. Patients may be advised that taking ropinirole tablets USP with food may reduce the occurrence of nausea. However, this has not been established in controlled clinical trials.
If a significant interruption in therapy with ropinirole tablets USP has occurred, retitration of therapy may be warranted.
Geriatric Use:
Pharmacokinetic studies demonstrated a reduced clearance of ropinirole in the elderly (see CLINICAL PHARMACOLOGY). Dose adjustment is not necessary since the dose is individually titrated to clinical response.
Renal Impairment:
The pharmacokinetics of ropinirole were not altered in patients with moderate renal impairment (see CLINICAL PHARMACOLOGY). Therefore, no dosage adjustment is necessary in patients with moderate renal impairment. The use of ropinirole tablets USP in patients with severe renal impairment has not been studied.
Hepatic Impairment:
The pharmacokinetics of ropinirole have not been studied in patients with hepatic impairment. Since patients with hepatic impairment may have higher plasma levels and lower clearance, ropinirole tablets USP should be titrated with caution in these patients.
Dosing for Parkinson’s Disease:
In all clinical studies, dosage was initiated at a subtherapeutic level and gradually titrated to therapeutic response. The dosage should be increased to achieve a maximum therapeutic effect, balanced against the principal side effects of nausea, dizziness, somnolence, and dyskinesia.
The recommended starting dose for Parkinson’s Disease is 0.25 mg 3 times daily. Based on individual patient response, dosage should then be titrated with weekly increments as described in Table 5. After week 4, if necessary, daily dosage may be increased by 1.5 mg/day on a weekly basis up to a dose of 9 mg/day, and then by up to 3 mg/day weekly to a total dose of 24 mg/day. Doses greater than 24 mg/day have not been tested in clinical trials.
| Week | Dosage | Total Daily Dose |
|
1 2 3 4 |
0.25 mg 3 times daily 0.5 mg 3 times daily 0.75 mg 3 times daily 1 mg 3 times daily |
0.75 mg 1.5 mg 2.25 mg 3 mg |
When ropinirole tablets USP are administered as adjunct therapy to L-dopa, the concurrent dose of L-dopa may be decreased gradually as tolerated. L-dopa dosage reduction was allowed during the advanced Parkinson’s disease (with L-dopa) study if dyskinesias or other dopaminergic effects occurred. Overall, reduction of L-dopa dose was sustained in 87% of patients treated with ropinirole hydrochloride and in 57% of patients on placebo. On average the L-dopa dose was reduced by 31% in patients treated with ropinirole hydrochloride.
Ropinirole tablets USP for Parkinson’s disease patients should be discontinued gradually over a 7-day period. The frequency of administration should be reduced from 3 times daily to twice daily for 4 days. For the remaining 3 days, the frequency should be reduced to once daily prior to complete withdrawal of ropinirole tablets USP.
Dosing for Restless Legs Syndrome:
In all clinical trials, the dose for ropinirole tablets USP was initiated at 0.25 mg once daily, 1 to 3 hours before bedtime. Patients were titrated based on clinical response and tolerability.
The recommended adult starting dosage for RLS is 0.25 mg once daily, 1 to 3 hours before bedtime. After 2 days, the dosage can be increased to 0.5 mg once daily and to 1 mg once daily at the end of the first week of dosing, then as shown in Table 6 as needed to achieve efficacy. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established.
| Day/Week | Dosage to be taken once daily,1 to 3 hours before bedtime |
| Days 1 and 2 | 0.25 mg |
| Days 3-7 | 0.5 mg |
| Week 2 | 1 mg |
| Week 3 | 1.5 mg |
| Week 4 | 2 mg |
| Week 5 | 2.5 mg |
| Week 6 | 3 mg |
| Week 7 | 4 mg |
In clinical trials of patients being treated for RLS with doses up to 4 mg once daily, ropinirole hydrochloride was discontinued without a taper.
HOW SUPPLIED
Tablets: Each tablet contains ropinirole hydrochloride USP
2 mg: pale pink to pink, circular, beveled edged, biconvex film coated tablets with ‘256’ debossed on one side and ‘G’ on the other side
NDC 68258-7163-03
STORAGE: Protect from light and moisture. Close container tightly after each use. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
SINEMET is a registered trademark of Merck & Co., Inc.
Manufactured by:
Glenmark Generics Ltd.
Colvale-Bardez, Goa 403 513, India
Manufactured for:

Glenmark Generics Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkgenerics.com
December 2011
PATIENT INFORMATION - Restless Legs Syndrome
rOPINIRole Tablets USP
If you have Restless Legs Syndrome (RLS, also known as Ekbom Syndrome), read this side
Read this information completely before you start taking ropinirole tablets. Read the information each time you get more medicine. There may be new information. This leaflet provides a summary about ropinirole tablets. It does not include everything there is to know about your medicine. This information should not take the place of discussions with your doctor about your medical condition or ropinirole tablets.
What are ropinirole tablets?
Ropinirole tablets are a prescription medicine to treat moderate-to-severe primary Restless Legs Syndrome. They are sometimes used to treat Parkinson's disease. Having one of these conditions does not mean you have or will develop the other.
What is the most important information I should know about ropinirole tablets?
- Patients with RLS should take ropinirole tablets differently than patients with Parkinson's disease (see How should I take ropinirole tablets for RLS? for the recommended dosing for RLS). A lower dose of ropinirole tablets is generally needed for patients with RLS, and is taken once daily before bedtime.
- There are known side effects of ropinirole tablets. If you fall asleep or feel very sleepy while doing normal activities such as driving, faint, feel dizzy, nauseated, or sweaty when you stand up from sitting or lying down, you should talk with your doctor (see What are the possible side effects of ropinirole tablets? ).
- Before starting ropinirole tablets, be sure to tell your doctor if you are taking any medicines that make you drowsy.
Who should not take ropinirole tablets?
You should not take ropinirole tablets if you are allergic to the active ingredient ropinirole or to any of the inactive ingredients. Your doctor and pharmacist have a list of the inactive ingredients.
What should I tell my doctor?
Be sure to tell your doctor if:
- you are pregnant or plan to become pregnant.
- you are breast-feeding.
- you have daytime sleepiness from a sleep disorder other than RLS or have unexpected sleepiness or periods of sleep while taking ropinirole tablets.
- you are taking any other prescription or over-the-counter medicines. Some of these medicines may increase your chances of getting side effects while taking ropinirole tablets.
- you start or stop taking other medicines while you are taking ropinirole tablets. This may increase your chances of getting side effects.
- you start or stop smoking while you are taking ropinirole tablets. Smoking may decrease the treatment effect of ropinirole tablets.
- you feel dizzy, nauseated, sweaty, or faint when you stand up from sitting or lying down.
- you drink alcoholic beverages. This may increase your chances of becoming drowsy or sleepy while taking ropinirole tablets.
How should I take ropinirole tablets for RLS?
- Be sure to take ropinirole tablets exactly as directed by your doctor or healthcare provider.
- The usual way to take ropinirole tablets is once in the evening, 1 to 3 hours before bedtime.
- Your doctor will start you on a low dose of ropinirole tablets. Your doctor may change the dose until you are taking the amount of medicine that is right for you to control your symptoms.
- You may receive a starting kit with doses marked by day. The pills in this kit slowly increase your daily dose over time so that you and your doctor may determine what the best dose is for you. Different people respond differently to this medicine. You may not need the highest dose pill in this kit or you may need an even higher dose to relieve your symptoms. You should carefully follow your doctor’s advice on the use of this kit.
- If you miss your dose, do not double your next dose. Take only your usual dose 1 to 3 hours before your next bedtime.
- Contact your doctor, if you stop taking ropinirole tablets for any reason. Do not restart without consulting your doctor.
- You can take ropinirole tablets with or without food. Taking ropinirole tablets with food may decrease the chances of feeling nauseated.
What are the possible side effects of ropinirole tablets?
- Most people who take ropinirole tablets tolerate them well. The most commonly reported side effects in people taking ropinirole tablets for RLS are nausea, vomiting, dizziness, and drowsiness or sleepiness. You should be careful until you know if ropinirole tablets affect your ability to remain alert while doing normal daily activities, and you should watch for the development of significant daytime sleepiness or episodes of falling asleep. It is possible that you could fall asleep while doing normal activities such as driving a car, doing physical tasks, or using hazardous machinery while taking ropinirole tablets. Your chances of falling asleep while doing normal activities while taking ropinirole tablets are greater if you are taking other medicines that cause drowsiness.
- When you start taking ropinirole tablets or when you increase your dose, you may feel dizzy, nauseated, sweaty or faint, when first standing up from sitting or lying down. Therefore, do not stand up quickly after sitting or lying down, particularly if you have been sitting or lying down for a long period of time. Take a minute sitting on the edge of the bed or chair before you get up.
- Some patients taking ropinirole have shown urges to behave in a way unusual for them. Examples of this are an unusual urge to gamble or increased sexual urges and/or behaviors. If you or your family notices that you are developing any unusual behaviors, talk to your doctor.
- Hallucinations (unreal sounds, visions, or sensations) have been reported in patients taking ropinirole tablets. These were uncommon in patients taking ropinirole tablets for RLS. The risk is greater in patients with Parkinson's disease who are elderly, taking ropinirole tablets with L-dopa, or taking higher doses of ropinirole tablets than recommended for RLS.
This is not a complete list of side effects and should not take the place of discussions with your healthcare providers. Your doctor or pharmacist can give you a more complete list of possible side effects. Talk to your doctor about any side effects or problems you may have.
Other Information about ropinirole tablets
- Studies of people with Parkinson’s disease show that they may be at an increased risk of developing melanoma, a form of skin cancer, when compared to people without Parkinson’s disease. It is not known if this problem is associated with Parkinson’s disease or the medicines used to treat Parkinson’s disease. Ropinirole tablets are one of the medicines used to treat Parkinson’s disease, therefore, patients being treated with ropinirole tablets should have periodic skin examinations.
- Take ropinirole tablets exactly as your doctor prescribes them.
- Do not share ropinirole tablets with other people, even if they have the same symptoms you have.
- Keep ropinirole tablets out of the reach of children.
- Store ropinirole tablets at room temperature out of direct sunlight.
- Keep ropinirole tablets in a tightly closed container.
This leaflet summarizes important information about ropinirole tablets. Medicines are sometimes prescribed for purposes other than those listed in this leaflet. Do not take ropinirole tablets for a condition for which it was not prescribed. For more information, talk with your doctor or pharmacist. They can give you information about ropinirole tablets that is written for healthcare professionals.
Manufactured by:
Glenmark Generics Ltd.
Colvale-Bardez, Goa 403 513, India
Manufactured for:

Glenmark Generics Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkgenerics.com
December 2011
PATIENT INFORMATION - Parkinson's Disease
rOPINIRole Tablets USP
If you have Parkinson's disease, read this side
Read this information completely before you start taking ropinirole tablets. Read the information each time you get more medicine. There may be new information. This leaflet provides a summary about ropinirole tablets. It does not include everything there is to know about your medicine. This information should not take the place of discussions with your doctor about your medical condition or ropinirole tablets.
What are ropinirole tablets?
Ropinirole tablets are a prescription medicine used to treat Parkinson's disease. They are also used to treat moderate-to-severe primary Restless Legs Syndrome. Having one of these conditions does not mean you have or will develop the other.
What is the most important information I should know about ropinirole tablets?
- Patients with Parkinson's disease should take ropinirole tablets differently than patients with Restless Legs Syndrome (see How should I take ropinirole tablets for Parkinson's disease? ) For Parkinson's disease, a higher dose of ropinirole tablets is generally needed, and is taken more frequently throughout the day.
- There are known side effects of ropinirole tablets (see What are the possible side effects of ropinirole tablets?)
- If you fall asleep or feel very sleepy while doing normal activities such as driving, faint, feel dizzy, nauseated, or sweaty when you stand up from sitting or lying down, you should talk with your doctor.
- Hallucinations (unreal visions, sounds, or sensations) have been reported in patients taking ropinirole tablets. The risk is greater in patients with Parkinson's disease who are elderly, taking ropinirole tablets with L-dopa or taking higher doses of ropinirole tablets. If these occur, you should discuss them with your doctor.
- Ropinirole tablets may make some of the side effects of L-dopa worse. Ropinirole tablets may cause uncontrolled sudden movements or make such movements you already have worse or more frequent. You should notify your doctor in such a case as dosage adjustments to your anti-Parkinson’s medications may be necessary.
- Before starting ropinirole tablets, be sure to tell your doctor if you are taking any medicines that make you drowsy.
Who should not take ropinirole tablets?
You should not take ropinirole tablets if you are allergic to the active ingredient ropinirole or to any of the inactive ingredients. Your doctor and pharmacist have a list of the inactive ingredients.
What should I tell my doctor?
Be sure to tell your doctor if:
- you are pregnant or plan to become pregnant.
- you are breast-feeding.
- you have daytime sleepiness from a sleep disorder or have unexpected sleepiness or periods of sleep while taking ropinirole tablets.
- you are taking any other prescription or over-the-counter medicines. Some of these medicines may increase your chances of getting side effects while taking ropinirole tablets.
- you start or stop taking other medicines while you are taking ropinirole tablets. This may increase your chances of getting side effects.
- you start or stop smoking while you are taking ropinirole tablets. Smoking may decrease the treatment effect of ropinirole tablets.
- you feel dizzy, nauseated, sweaty, or faint when you first stand up from sitting or lying down.
- you drink alcoholic beverages. This may increase your chances of becoming drowsy or sleepy while taking ropinirole tablets.
How should I take ropinirole tablets for Parkinson's disease?
- Be sure to take your ropinirole tablets exactly as directed by your doctor or healthcare provider.
- Three times a day is the usual way to take ropinirole tablets for Parkinson's disease.
- Your doctor will start you on a low dose of ropinirole tablets. Your doctor will change the dose until you are taking the right amount of medicine to control your symptoms. It may take several weeks before you reach a dose that controls your symptoms.
- If you miss a dose, do not double your next dose.
- Contact your doctor, if you stop taking ropinirole tablets for any reason. Do not restart without consulting your doctor.
- Your doctor may prescribe ropinirole tablets alone or add ropinirole tablets to medicine that you are already taking for Parkinson's disease.
- You can take ropinirole tablets with or without food. Taking ropinirole tablets with food may decrease the chances of feeling nauseated.
What are the possible side effects of ropinirole tablets?
- Most people who take ropinirole tablets tolerate them well. The most commonly reported side effects in people taking ropinirole tablets are nausea, headache, dizziness, drowsiness or sleepiness.
- You should be careful until you know if ropinirole tablets affect your ability to remain alert while doing normal daily activities, and you should watch for the development of significant daytime sleepiness or episodes of falling asleep. It is possible that you could fall asleep while doing normal activities such as driving a car, doing physical tasks, or using hazardous machinery while taking ropinirole tablets. Your chances of falling asleep while doing normal activities while taking ropinirole tablets are greater if you are taking other medicines that cause drowsiness.
- When you start taking ropinirole tablets or when you increase your dose, you may feel dizzy, nauseated, sweaty or faint, when first standing up from sitting or lying down. Therefore, do not stand up quickly after sitting or lying down, particularly if you have been sitting or lying down for a long period of time. Take a minute sitting on the edge of the bed or chair before you get up.
- Some patients taking ropinirole have shown urges to behave in a way unusual for them. Examples of this are an unusual urge to gamble or increased sexual urges and/or behaviors. If you or your family notices that you are developing any unusual behaviors, talk to your doctor.
- Hallucinations (unreal visions, sounds, or sensations) have been reported in patients taking ropinirole tablets. The risk is greater in patients with Parkinson's disease who are elderly, taking ropinirole tablets with L-dopa, or taking higher amounts of ropinirole tablets.
- If you are taking L-dopa for Parkinson's disease, ropinirole tablets may make some of the side effects of L-dopa worse. Ropinirole tablets may cause uncontrolled sudden movements or make such movements you already have worse or more frequent.
This is not a complete list of side effects and should not take the place of discussions with your healthcare providers. Your doctor or pharmacist can give you a more complete list of possible side effects. Talk to your doctor about any side effects or problems you may have.
Other Information about ropinirole tablets
- Studies of people with Parkinson’s disease show that they may be at an increased risk of developing melanoma, a form of skin cancer, when compared to people without Parkinson’s disease. It is not known if this problem is associated with Parkinson’s disease or the medicines used to treat Parkinson’s disease. Therefore, patients being treated with ropinirole tablets should have periodic skin examinations.
- Take ropinirole tablets exactly as your doctor prescribes them.
- Do not share ropinirole tablets with other people, even if they have the same symptoms you have.
- Keep ropinirole tablets out of the reach of children.
- Store ropinirole tablets at room temperature out of direct sunlight.
- Keep ropinirole tablets in a tightly closed container.
This leaflet summarizes important information about ropinirole tablets. Medicines are sometimes prescribed for purposes other than those listed in this leaflet. Do not take ropinirole tablets for a condition for which it was not prescribed. For more information, talk with your doctor or pharmacist. They can give you information about ropinirole tablets that is written for healthcare professionals.
Manufactured by:
Glenmark Generics Ltd.
Colvale-Bardez, Goa 403 513, India
Manufactured for:

Glenmark Generics Inc., USA
Mahwah, NJ 07430
Questions? 1 (888)721-7115
www.glenmarkgenerics.com
December 2011
Principal Display Panel
NDC 68258-7163-XX
NDC 68258-7163-03
ropiniroleropinirole TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||